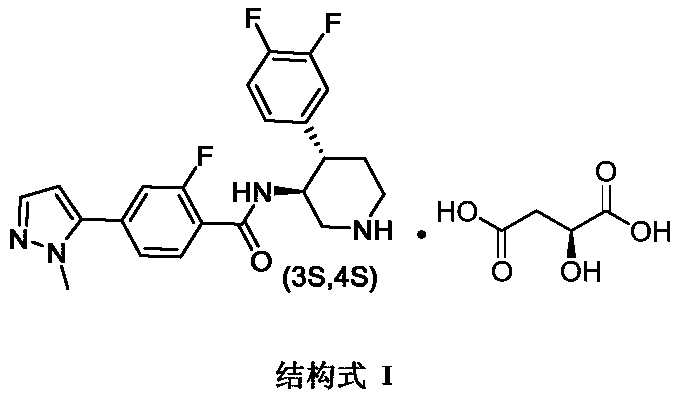
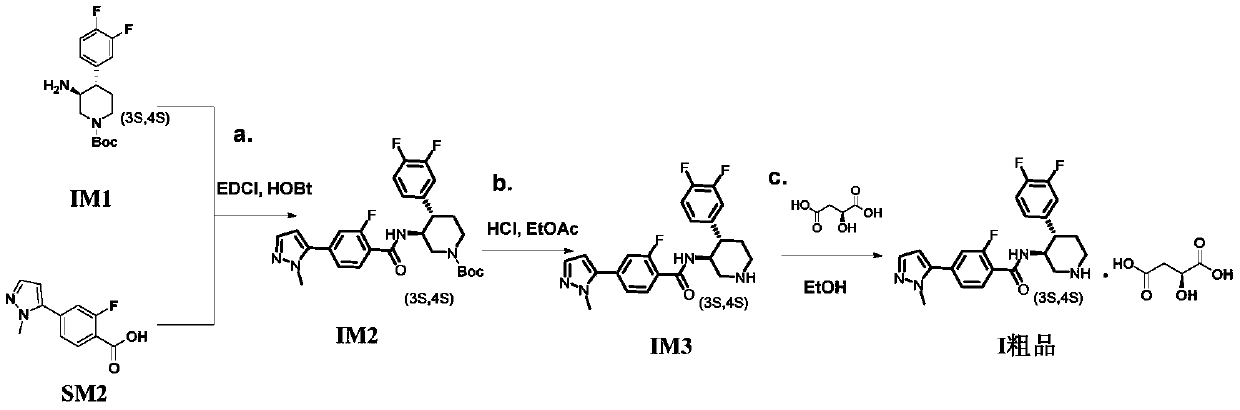
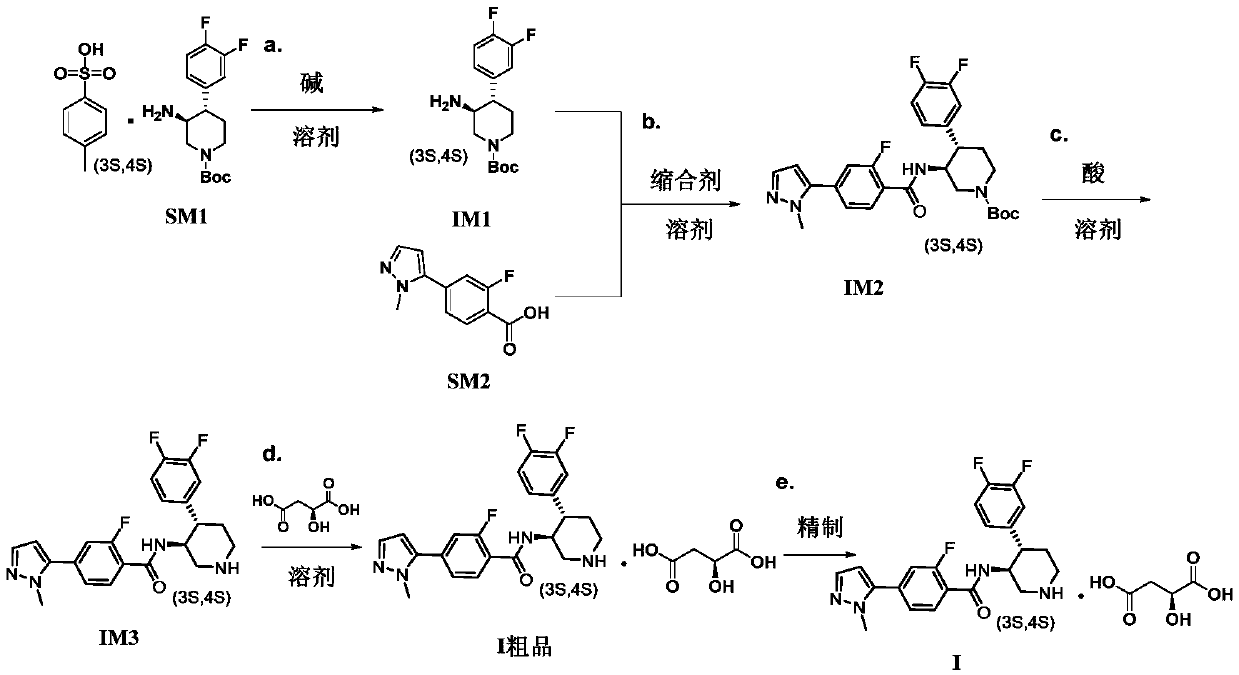
A kind of preparation technology of polyfluorine-substituted aromatic heterocyclic compounds
A preparation process and a technology for heterocycles are applied in the field of pharmaceutical synthesis to achieve the effects of high stability, high yield and easy preservation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] Preparation of starting compound SM1:
[0041]
[0042] Compound SM1 used in this example can be prepared by the following method:
[0043] 172.32 g of compound IM1 (1.0 eq) was added to a 1 L reaction flask, and 344 ml of absolute ethanol was added thereto. 93.3g of p-toluenesulfonic acid monohydrate (1.0eq) was dissolved in 172ml of ethanol, and then added to the reaction solution under ice-bath conditions, and the dropwise addition was completed within 1h. During the dropwise addition, when a large amount of solids appeared in the reaction solution and could not be stirred, 340ml of MTBE was added and the reaction was continued for 30min. After the reaction was finished, it was filtered with suction, and the filter cake was washed with 100 ml of MTBE×5 to obtain 178 g of intermediate SM1. Intermediate 11 was reduced and salt-formed to obtain intermediate SM1, and the total yield of the two-step reaction was 74.86%. 1 H NMR (500MHz, DMSO-d 6 )δ7.75(s,2H),7.53–7...
Embodiment 1
[0047] Embodiment 1: the preparation of intermediate IM1
[0048]
[0049] 160g of compound SM1 (1.0eq) (ee value 99.8%) was suspended in 1.6L of dichloromethane, stirred at room temperature, 727ml of 0.5mol / L NaOH solution (1.1eq) was added dropwise, stirred at room temperature for 30min, the reaction solution It became clear, and the layers were separated after standing. The aqueous layer was extracted once again with 200ml of DCM. The combined organic layers were washed with 100ml of saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure at 40°C to obtain 108.28g of golden yellow oily liquid. The yield of body IM1 is 104.86% based on crude product IM1.
[0050] 1 H NMR (400MHz, CDCl 3 )δ7.11(dd, J=18.5,8.3Hz,1H),7.06-7.00(m,1H),6.97–6.91(m,1H),4.23(d,J=25.4Hz,2H),2.80(d ,J=42.4Hz,2H),2.50(t,J=11.3Hz,1H),2.38–2.25(m,1H),1.76(d,J=11.4Hz,1H),1.68-1.57(m,1H) ,1.47(s,9H),1.45-1.41(m,2H).
Embodiment 2
[0051] Embodiment 2: the preparation of intermediate IM1
[0052] Suspend 0.5 g of SM1 (1.0 eq) in 5 ml of dichloromethane, add 2.1 ml of 0.5 mol / L NaOH aqueous solution (1.0 eq) to the reaction solution, complete the addition within 3 min, and stir at room temperature for 1 h. After static separation, the DCM layer was washed with 5ml of saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure at 40°C to obtain 0.282g of intermediate IM1 as a golden oily liquid, with a yield of 98.75% based on the crude product IM1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com