Synthesis method of 3, 5-diaryl-4-trifluoromethyl pyrazole derivative
A technology of trifluoromethylpyrazole and a synthesis method, applied in 3 fields, can solve the problems of low metal catalysis economy, troubled multi-step reaction, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
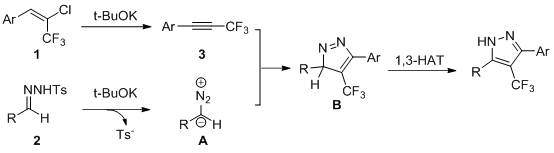
[0023] Specific example one: 41.2 mg (0.2 mmol) 2-chloro-3,3,3-trifluorophenylpropene, 65.8 mg (0.24 mmol) benzaldehyde p-toluenesulfonyl hydrazone, 67.3 mg (0.6 mmol) tert-butanol Potassium was added to 3 ml of solvent tetrahydrofuran. The reaction was stirred at 70 °C for 12 hours. Cool after the reaction, filter the reaction solution to obtain the filtrate and wash it with saturated sodium chloride solution, extract it with ethyl acetate and dry it with anhydrous magnesium sulfate, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, and pass the residue through a silica gel column with The eluent prepared by petroleum ether and ethyl acetate at a volume ratio of 10:1 was used for eluting, the effluent was collected according to the actual gradient, and detected by TLC, the effluent containing the target product was combined, and the combined effluent was used with a rotary evaporator The solvent was removed by rotation, and dried in vac...
specific Embodiment 2
[0024] Specific example two: 44 mg (0.2 mmol) 1-(2-chloro-3,3,3-trifluoro-1-propenyl)-4-toluene, 65.8 mg (0.24 mmol) benzaldehyde p-toluenesulfonyl Hydrazone, 67.3 mg (0.6 mmol) of potassium tert-butoxide, was added to 3 ml of solvent tetrahydrofuran. The reaction was stirred at 70 °C for 12 hours. Cool after the reaction, filter the reaction solution to obtain the filtrate and wash it with saturated sodium chloride solution, extract it with ethyl acetate and dry it with anhydrous magnesium sulfate, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, and pass the residue through a silica gel column with The eluent prepared by petroleum ether and ethyl acetate at a volume ratio of 10:1 was used for eluting, the effluent was collected according to the actual gradient, and detected by TLC, the effluent containing the target product was combined, and the combined effluent was used with a rotary evaporator The solvent was removed by rotation an...
specific Embodiment 3
[0025] Specific example three: 44 mg (0.2 mmol) 1-(2-chloro-3,3,3-trifluoro-1-propenyl)-2-methylbenzene, 65.8 mg (0.24 mmol) benzaldehyde p-toluene Sulfonylhydrazone, 67.3 mg (0.6 mmol) of potassium tert-butoxide, was added to 3 ml of solvent tetrahydrofuran. The reaction was stirred at 70 °C for 12 hours. Cool after the reaction, filter the reaction solution to obtain the filtrate and wash it with saturated sodium chloride solution, extract it with ethyl acetate and dry it with anhydrous magnesium sulfate, and use a rotary evaporator to remove the solvent from the filtrate to obtain a residue, and pass the residue through a silica gel column with The eluent prepared by petroleum ether and ethyl acetate at a volume ratio of 10:1 was used for eluting, the effluent was collected according to the actual gradient, and detected by TLC, the effluent containing the target product was combined, and the combined effluent was used with a rotary evaporator The solvent was removed by rot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com