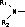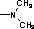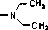Pleuromutilin derivative with thiadiazole skeleton, as well as preparation method and applications thereof
A technology of pleuromutilin and thiadiazole is applied in the field of pleuromutilin derivatives and their preparation, and can solve the problems of low activity, low antibacterial activity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Synthesis of 22-O-(4-toluenesulfonyl)oxyacetylmpirin (2)
[0072] Dissolve 75.7g (0.2mol) of pleuromutilin and 42g (0.22mol) of p-toluenesulfonyl chloride in 200ml of methyl tert-butyl ether, stir the mixture slowly and drop into NaOH solution with a concentration of 0.01mol / ml 50ml. Then heated to reflux while vigorously stirring. After 10-20min, a large amount of white matter was formed, and then stirred for 20-40min. Filter while hot with a Buchner funnel, then rinse with methyl tert-butyl ether (MTBE) and distilled water, and dry naturally to obtain a white powder product (2), with a yield of 97.8%.
[0073] mp 147~148 oC; IR (KBr): 3446, 2924, 2863, 1732, 1633, 1597, 1456, 1371, 1297, 1233, 1117, 1035, 832, 664, 560 cm-1; 1H NMR (400 MHz, CDCl3) δ 0.63 (d, 3H, J = 6.8 Hz), 0.87 (d, 3H, J = 6.8Hz), 1.11–1.15 (m, 1H), 1.22-1.26 (s, 5H), 1.33–1.36 (m , 1H), 1.41–1.44 (m, 1H), 1.46-1.50 (m, 5H), 1.63-1.65 (dd, 2H,J1=10Hz,J2=7.2 Hz), 2.01–2.08(m, 3H), 2....
Embodiment 2
[0074] Example 2: Synthesis of 14-O-(iodoacetyl) Mutilin (3)
[0075] 7.2g of 22-O-(4-toluenesulfonyl)oxyacetylmpirin (13.5mmol) was dissolved in 200mL of acetone, 2.3g of KI (14mmol) was added, and the reaction was terminated after reflux for 1h. The reaction mixture was filtered to remove insoluble salts, the filtrate was concentrated to 20-30 mL and filtered, and the filtrate was directly recrystallized at room temperature to give 5.36 g of a light yellow target compound.
[0076]Pale yellow powder, yield 81%, mp: 117-119 oC; IR (KBr): 3307, 2979, 2933, 1736, 1712, 1456, 1417, 1279, 1084, 1023, 985, 967 cm-1; 1H NMR (400 MHz, CDCl3) δ 7.26 (s, 1H), 6.41 (dd, J = 17.4, 11.0 Hz, 1H), 5.70 (d, J = 8.5 Hz, 1H), 5.34 (d, J = 11.0 Hz, 1H), 5.20 (dd, J = 17.4, 1.1 Hz, 1H), 3.65 (d, J = 10.4 Hz, 1H), 3.57 (d, J = 10.5 Hz, 1H), 3.35 (s, 1H), 2.36 – 2.28 (m, 1H), 2.26 – 2.14 (m, 2H), 2.07 (dd, J = 16.0, 8.5 Hz, 2H), 1.79 – 1.60 (m, 4H), 1.57 – 1.47 (m, 2H), 1.47 – 1.42 (m, 4H), ...
Embodiment 3
[0077] Example 3: Synthesis of 14-O-[(2-amino-1,3,4,-thiadiazol-5-yl)thioacetyl]Multiline (Intermediate 3)
[0078] 2.45 g of 14-O-(iodoacetyl) Mutilin (5 mmol) was dissolved in 60 mL of THF, 0.73 g of 2-amino-5-mercapto-1,3,4,-thiadiazole (5.5 mmol) was dissolved in 5 mL of 10% NaOH and added dropwise to the above solution. mixture at 0 o C stirred for 2 h, then evaporated to dryness under reduced pressure, and added 100 mL of ethyl acetate. The resulting mixture was washed with saturated NH 4 Cl washed, and extracted 3 times with distilled water, separated the organic phase and washed with anhydrous Na 2 SO 4 After drying overnight, the filtrate was filtered and the mixture was separated on a silica gel column (petroleum ether: ethyl acetate = 1:2) to obtain 2.3 g of the target compound.
[0079] White powder, yield 92%, mp 74-75 o C.;IR (KBr): 3419, 3330, 2931, 1731, 1616, 1507, 1456, 1417, 1373, 1282, 1190, 1152, 1117, 1019, 980, 953, 938,916 cm -1 ; 1 H NMR (400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com