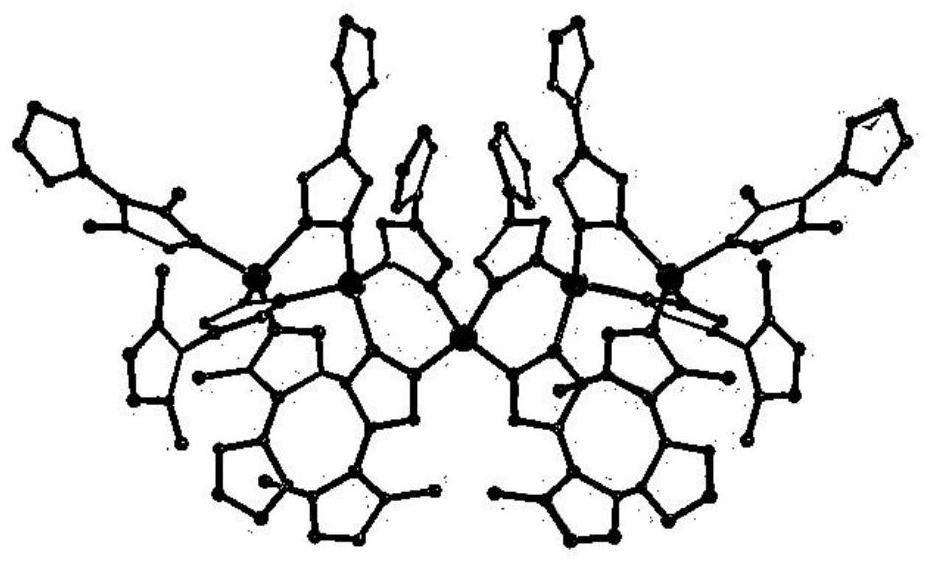
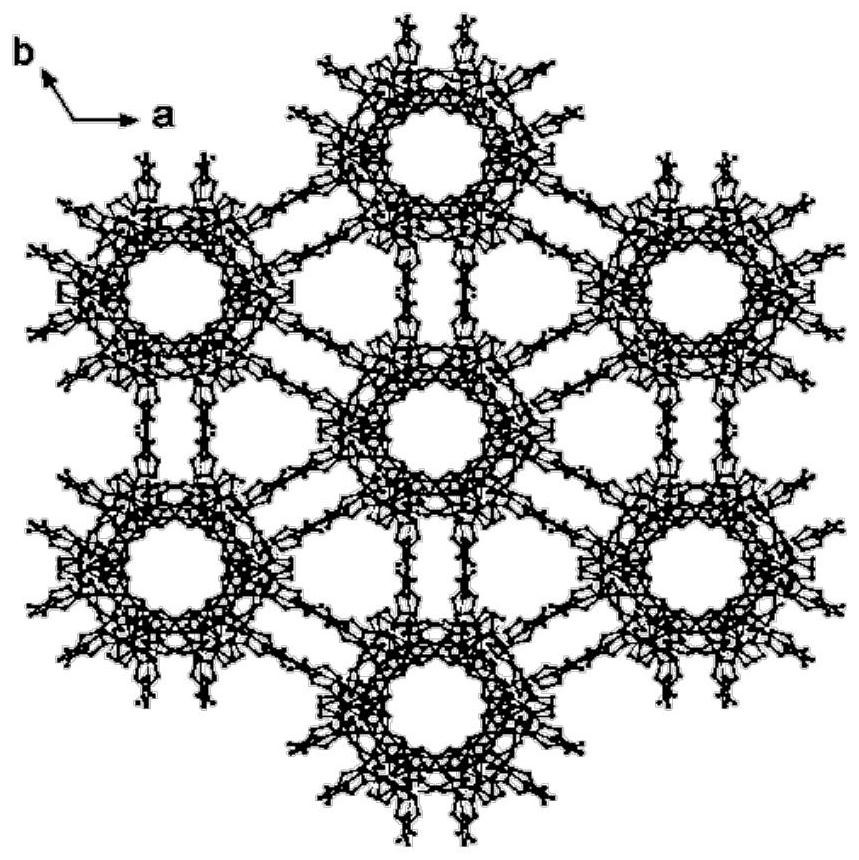
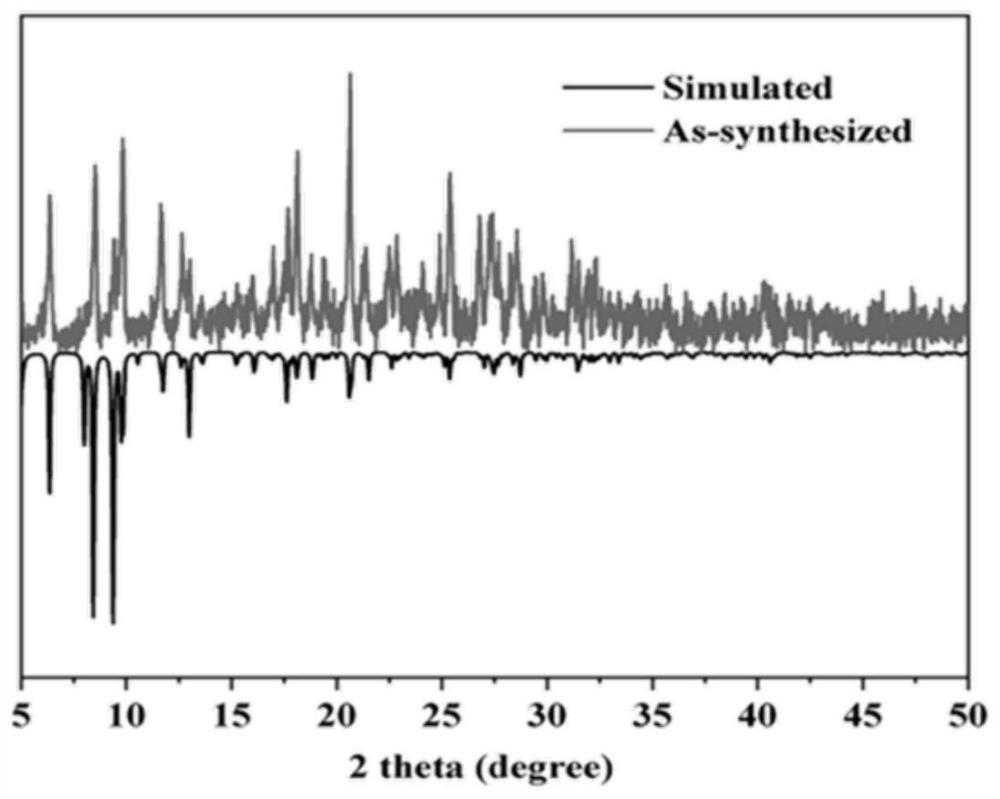
Metal-organic framework material as well as preparation method and application thereof
An organic framework and metal technology, applied in the field of metal-organic framework materials and their preparation, can solve the problems of low specific surface area, poor pollutant removal rate and adsorption capacity, difficult surface modification, etc., and achieve high thermal and chemical stability , Large adsorption capacity and high adsorption rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Synthesis of organic ligand 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr:
[0035] Synthesis of 4-amino-3,5-dimethylpyrazole ligand intermediate: 5.07g, 36mmol of 4-nitro-3,5-dimethylpyrazole and 40.0g KOH were dissolved in 100mL deionized water, Heat it to 90°C in an oil bath, weigh 27.57g, 122mmol of SnCl 2 2H 2 O, added to the above system in three times, the feeding interval is 1 hour, keep the temperature constant, continue to react for 6 hours, cool to room temperature, filter the solid product, wash with 3mL ice water, dry to get 4-amino-3,5 - 2.60 g of dimethylpyrazole ligand intermediate, the yield is about 65%;
[0036] Synthesis of 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr ligand: 3.33g, 30mmol of 4-amino-3,5-dimethylpyrazole , 4.69g, 33mmol of N,N'-bis(dimethylaminomethylene)hydrazine and 0.24g, 1.3mmol of p-toluenesulfonic acid were dissolved in 25mL of o-xylene, stirred and refluxed for 24h, after the reaction was completed, the The sy...
Embodiment 2
[0049] (1) Synthesis of organic ligand 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr:
[0050] Synthesis of 4-amino-3,5-dimethylpyrazole ligand intermediate: 5.07g, 36mmol of 4-nitro-3,5-dimethylpyrazole and 51.0g KOH were dissolved in 100mL deionized water, Heat it to 90°C in an oil bath, weigh 27.0g of SnCl 2 2H 2 O, added to the above system in three times, the feeding interval is 1 hour, keep the temperature constant, continue to react for 6 hours, cool to room temperature, filter the solid product, wash with 3mL ice water, dry to get 4-amino-3,5 - 2.10 g of dimethylpyrazole ligand intermediate, the yield is about 52%;
[0051] Synthesis of 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr ligand: 3.33g, 30mmol of 4-amino-3,5-dimethylpyrazole , 3.50g of N,N'-bis(dimethylaminomethylene)hydrazine and 0.16g of p-toluenesulfonic acid were dissolved in 25mL of o-xylene, stirred and refluxed for 24h, after the reaction was completed, the system was cooled to room temperat...
Embodiment 3
[0059] (1) Synthesis of organic ligand 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr:
[0060] Synthesis of 4-amino-3,5-dimethylpyrazole ligand intermediate: 5.07g, 36mmol of 4-nitro-3,5-dimethylpyrazole and 40.0g KOH were dissolved in 100mL deionized water, Heat it to 90°C in an oil bath, weigh 27.57g, 122mmol of SnCl 2 2H 2 O, added to the above system in three times, the feeding interval is 1 hour, keep the temperature constant, continue to react for 6 hours, cool to room temperature, filter the solid product, wash with 3mL ice water, dry to get 4-amino-3,5 - 2.60 g of dimethylpyrazole ligand intermediate, the yield is about 65%;
[0061] Synthesis of 4-(3,5-dimethylpyrazol-4-yl)-1,2,4-triazole Hpztr ligand: 3.33g, 30mmol of 4-amino-3,5-dimethylpyrazole , 4.69g 33mmol of N,N'-bis(dimethylaminomethylene)hydrazine and 0.24g, 1.3mmol of p-toluenesulfonic acid were dissolved in 25mL of o-xylene, stirred and refluxed for 24h, after the reaction was completed, the system ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com