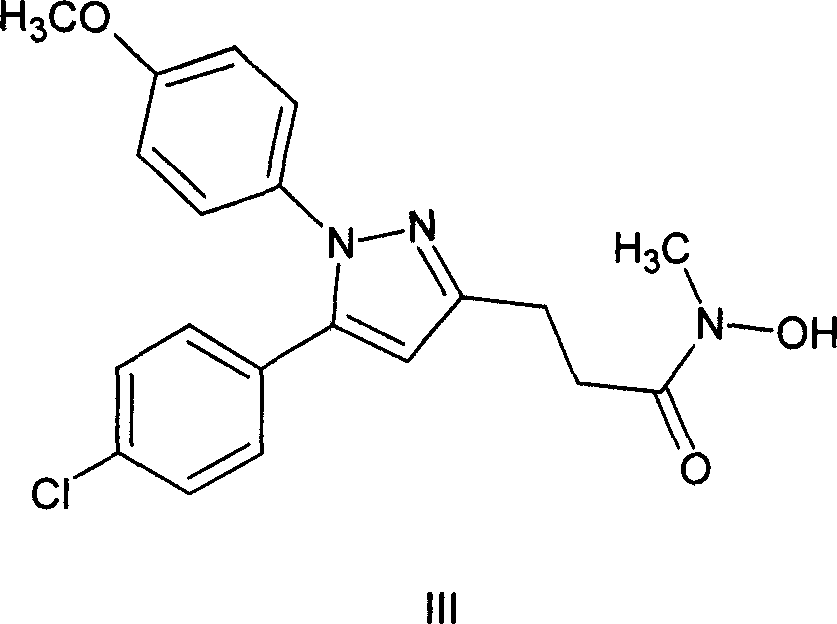
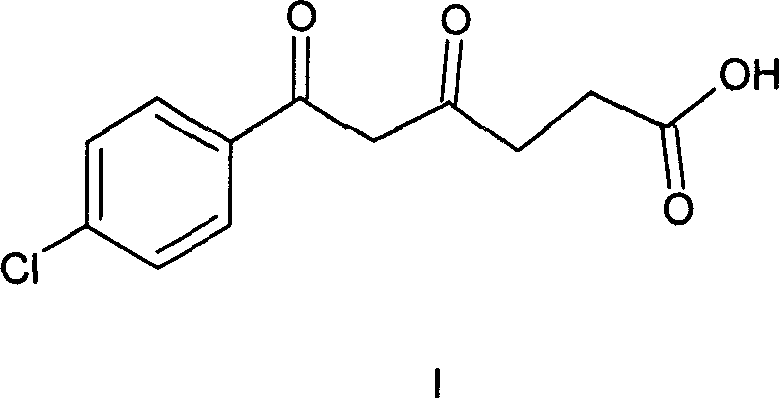
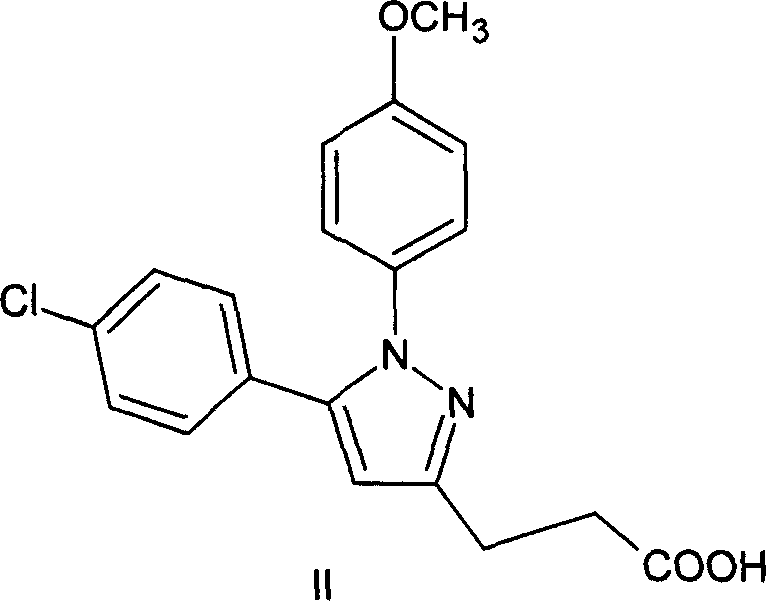
Method of synthesizing 5-(4-chloro-phenyl)-N-hydroxy-1-(4-methoxy-phenyl)-N-methyl-1H-pyrazole-3-propionamide and pharmaceutical use
A compound, butyl technology, applied in the field of organic compound synthesis, can solve problems such as high risk and easy combustion, and achieve the effects of simple steps, high safety, and elimination of environmental pollution problems.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0038] (1) Synthesis of 4-chloro-γ, ε-dioxo-phenylhexanoic acid (compound of formula I)
[0039] Potassium tert-butoxide (22.10 g, 0.20 mol) was dissolved in THF (210 mL) to make a solution. Dissolve p-chloroacetophenone (13.40 g, 0.09 mol) in THF (30 mL) at a temperature of -15-10° C., and add it dropwise to the above-mentioned THF solution of potassium tert-butoxide with a constant pressure funnel within 40 minutes. React for half an hour after dropping. Succinic anhydride (10.51g, 0.11mol) was dissolved in THF (170mL) to make a solution, and was added dropwise to the above reaction solution at a temperature of -15-10°C, and the drop was completed within 3.5 hours, and the reaction was continued for 2 hours.
[0040] At room temperature, the pH value of the reaction solution was adjusted to 4-5 with dilute hydrochloric acid (40 mL) (concentrated hydrochloric acid:water=1:7, volume ratio). The reaction solution was suction-filtered, and the filter cake was washed with a sma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com