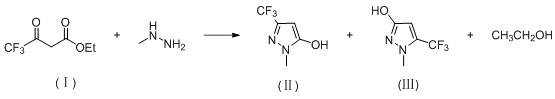
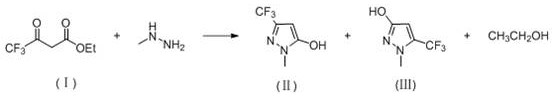
Synthesis method of high-selectivity 1-methyl-3-(trifluoromethyl)-1H-pyrazole-5-alcohol
A trifluoromethyl, high-selectivity technology, applied in organic chemistry and other directions, can solve problems such as poor selectivity, unfavorable industrial production, and violent reaction process, and achieve improved selectivity and yield, easy control of the reaction process, and reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 92 g (0.5 mol) 4,4-trifluoroacetate ethyl ester, 80 ml of ethanol and 0.92 gm cm-41 mesoporous molecular sieve catalyst were added to the reactor at room temperature, and the reaction system was maintained at 40 ° C, and then 69 g started ( 0.6 mol) 40% methylhydrazine solution, the control temperature was at 45 to 50 ° C, and the retained reagent was 50 ° C for 5 hours, the reaction was completed, filtered, a small amount of water washing filter, 79.31G1 - Methyl-3- (trifluoromethyl) -1H-pyrazole-5-alcohol, yield 95.55%; selectivity is 99.6: 0.4.
[0032] 1 H NMR (500 MHz, DMSO- d 6 δ 11.66 (S, 1H), 5.71 (S, 1H), 3.58 (S, 3H). 13 CNMR (126 MHz, DMSO- d 6 ) δ 153.07, 138.33 (q, 2 J F-C = 36.54 hz), 121.56 (q, 1 J F-C = 268.38 Hz), 84.41, 33.71.
Embodiment 2
[0034] 92 g (0.5 mol) 4,4,4-trifluoroacetate, 80 ml of ethanol and 0.92 g of ZSM-5 molecular sieve catalyst were added to the reactor at room temperature, and the reaction system was maintained at 40 ° C, and then 138 g (0.6 mol) 20% methylhydrazine aqueous solution, the control temperature was at 80 ° C after 5 hours after the measuring temperature of 45 ~ 50 ° C. The reaction was completed to cool to room temperature, filtered, a small amount of water washing filter cake, 74.64 g 1-methyl-3- (trifluoromethyl) -1H-pyrazole-5-alcohol, yield was 89.92%, selectivity was 99.1 : 0.9.
[0035]1 H NMR (500 MHz, DMSO- d 6 δ 11.66 (S, 1H), 5.71 (S, 1H), 3.58 (S, 3H). 13 CNMR (126 MHz, DMSO- d 6 ) δ 153.07, 138.33 (q, 2 J F-C = 36.54 hz), 121.56 (q, 1 J F-C = 268.38 Hz), 84.41, 33.71.
Embodiment 3
[0037] 92 g (0.5 mol) of 4,4,4-trifluoroacetate, 80 ml of ethanol and 0.92 gsba-15 molecular sieve catalyst were added to the reactor at room temperature, and the reaction system was maintained at 40 ° C, and then 69 g (0.6 mol) 40% methylhydrazine aqueous solution, the control temperature was at 45 to 50 ° C, and the maintenance system was reacted at 80 ° C after 5 hours after the drop was completed. The reaction ends for cooling to room temperature, filtration, small amount of water washing cake, 76.13 g 1-methyl-3- (trifluoromethyl) -1H-pyrazole-5-alcohol, yield 91.72%, selectivity is 99.2 : 0.8.
[0038] 1 H NMR (500 MHz, DMSO- d 6 δ 11.66 (S, 1H), 5.71 (S, 1H), 3.58 (S, 3H). 13 CNMR (126 MHz, DMSO- d 6 ) δ 153.07, 138.33 (q, 2 J F-C = 36.54 hz), 121.56 (q, 1 J F-C = 268.38 Hz), 84.41, 33.71.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com