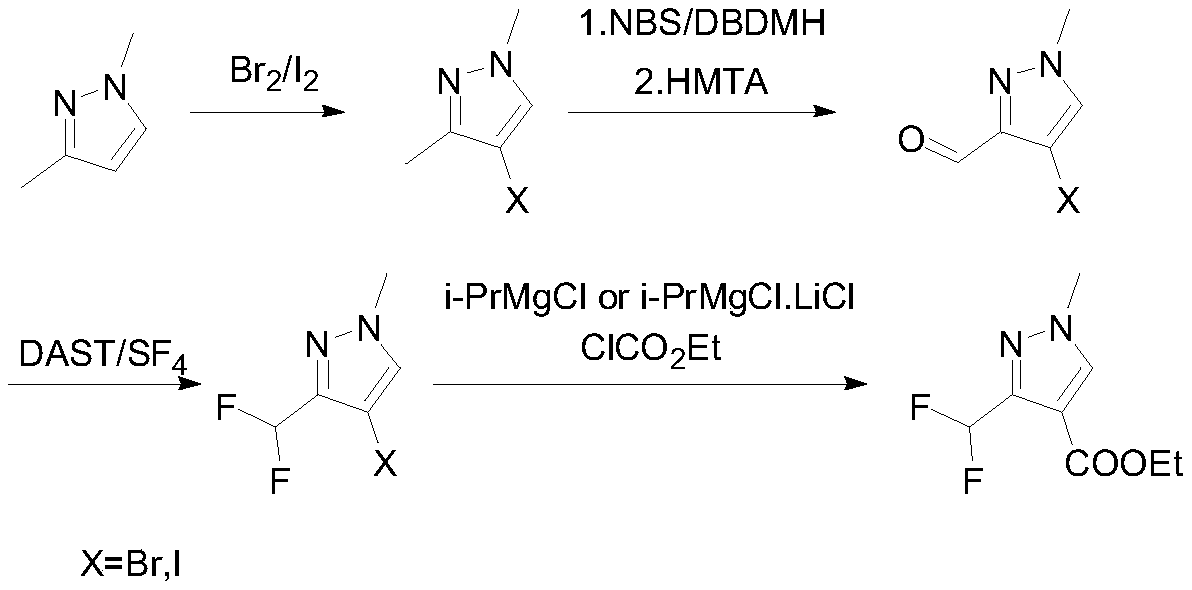
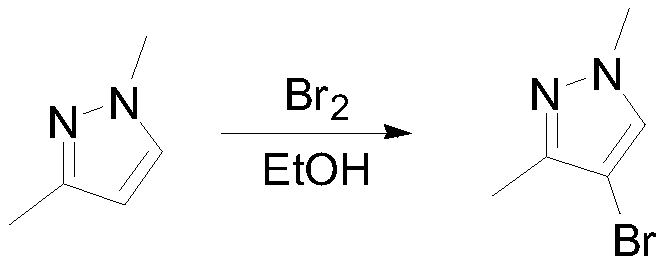Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester
A technology of dimethylpyrazole and difluoromethyl, which is applied in the field of preparation of ethyl 3--1-methyl-1H-pyrazole-4-carboxylate, can solve the problem of insufficient purity and the existence of isomers, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] 100g (1.04mol, 1eq) of 1,3-dimethylpyrazole and 400mL of ethanol were put into the reaction flask, and 166.4g (1.04mol, 1.00eq) of bromine was added dropwise with stirring at 5-10°C. React at 5-10°C for 4 hours, sample GC to detect the remaining raw materials 1 HNMR (400MHz, CDCl3): 7.97(s, 1H), 3.95(s, 3H), 2.77(s, 3H).
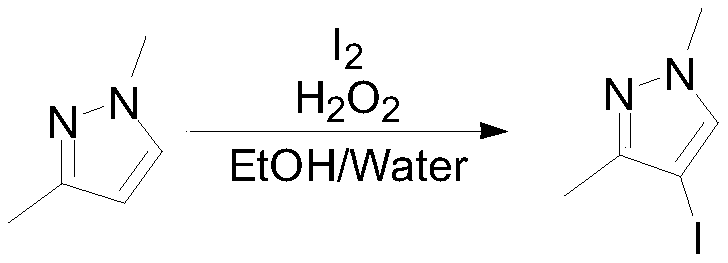
Embodiment 2
[0035]
[0036] Into the reaction flask, put 100g (1.04mol, 1eq) of 1,3-dimethylpyrazole, 800mL water and 400mL ethanol, add 132.1g (0.52mol, 0.5eq) of iodine in batches under stirring at room temperature, and then control the temperature at 84.8g (0.624mol, 0.6eq) of 25% hydrogen peroxide was added dropwise at 15-20°C. Stir at room temperature for 20 hours, sample GC to detect the remaining raw materials 1 HNMR (400MHz, CDCl 3 ):7.80(s,1H),3.95(s,3H),2.15(s,3H).
[0037] The second step: the synthesis of 4-(bromo / iodo)-1-methyl-1H-pyrazole-3-carbaldehyde
Embodiment 3
[0039]
[0040] 150g (0.857mol, 1eq) of 4-bromo-1,3-dimethyl-1H-pyrazole and 750mL of dichloromethane were put into the reaction flask, and 14g (0.086mol, 0.1 eq) Raise the temperature to 35-40°C and add NBS243.9g (1.37mol, 1.6eq) in batches. Subsequently, the temperature was controlled at 65-70° C. for 7 hours. Sampling GC to detect that the remaining raw material is 1 HNMR (400MHz, CDCl 3 ):9.75(s,1H),7.91(s,1H),3.95(s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com