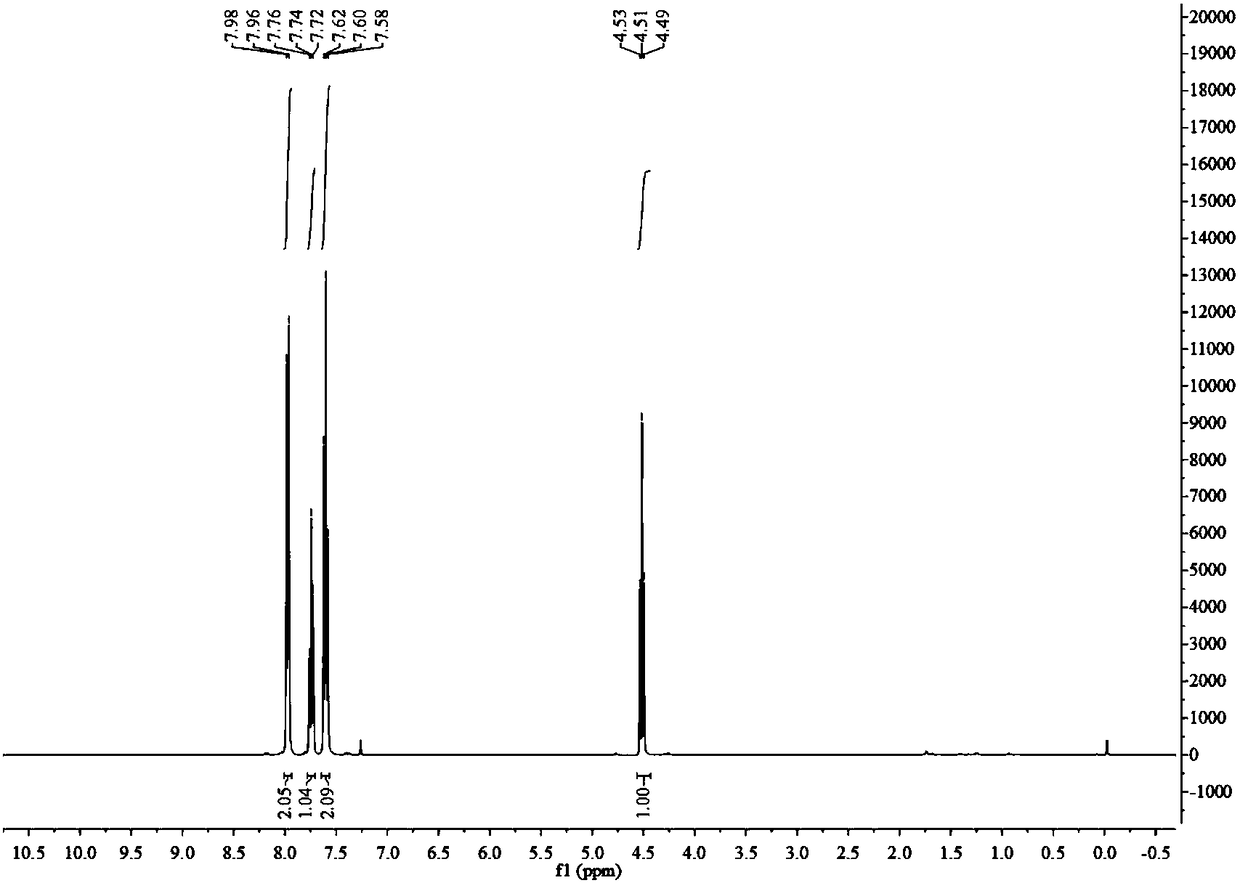
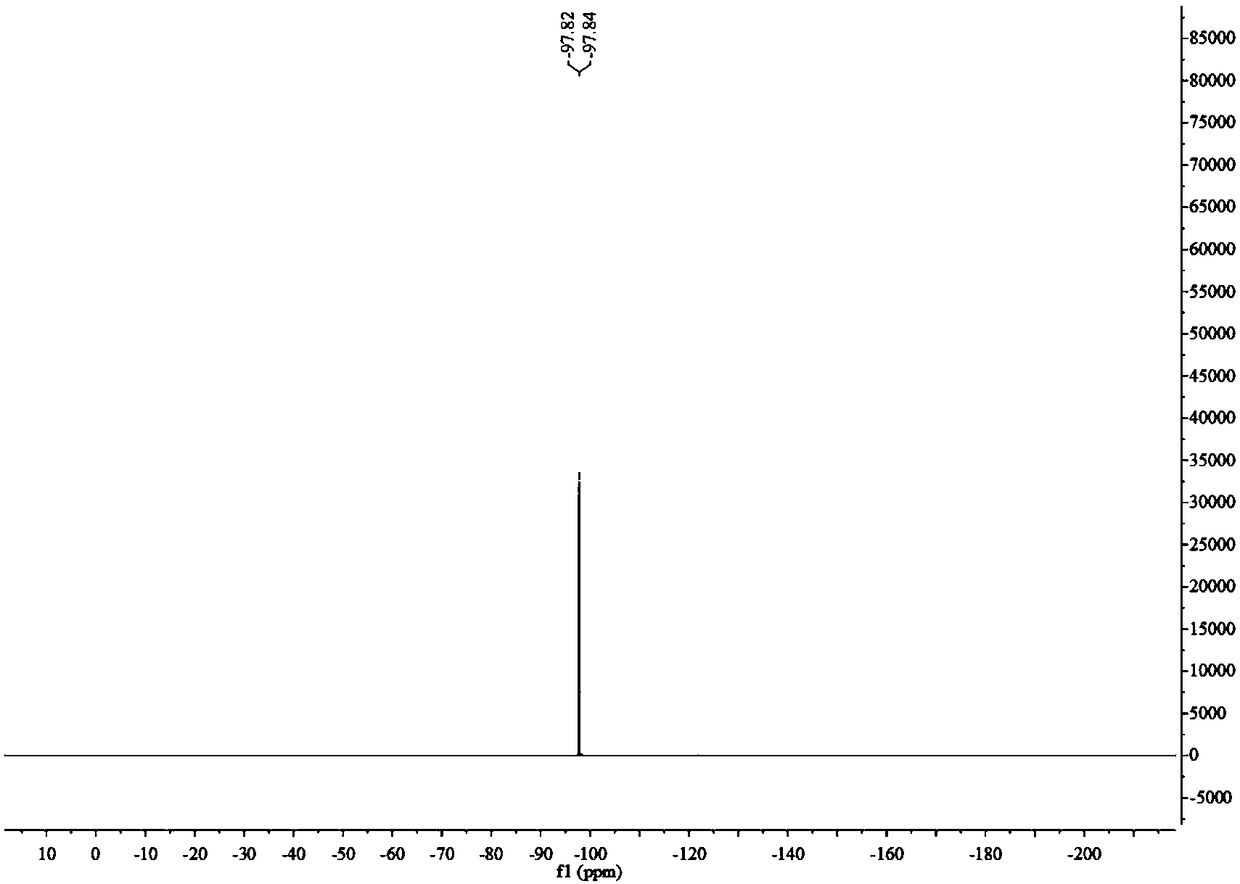
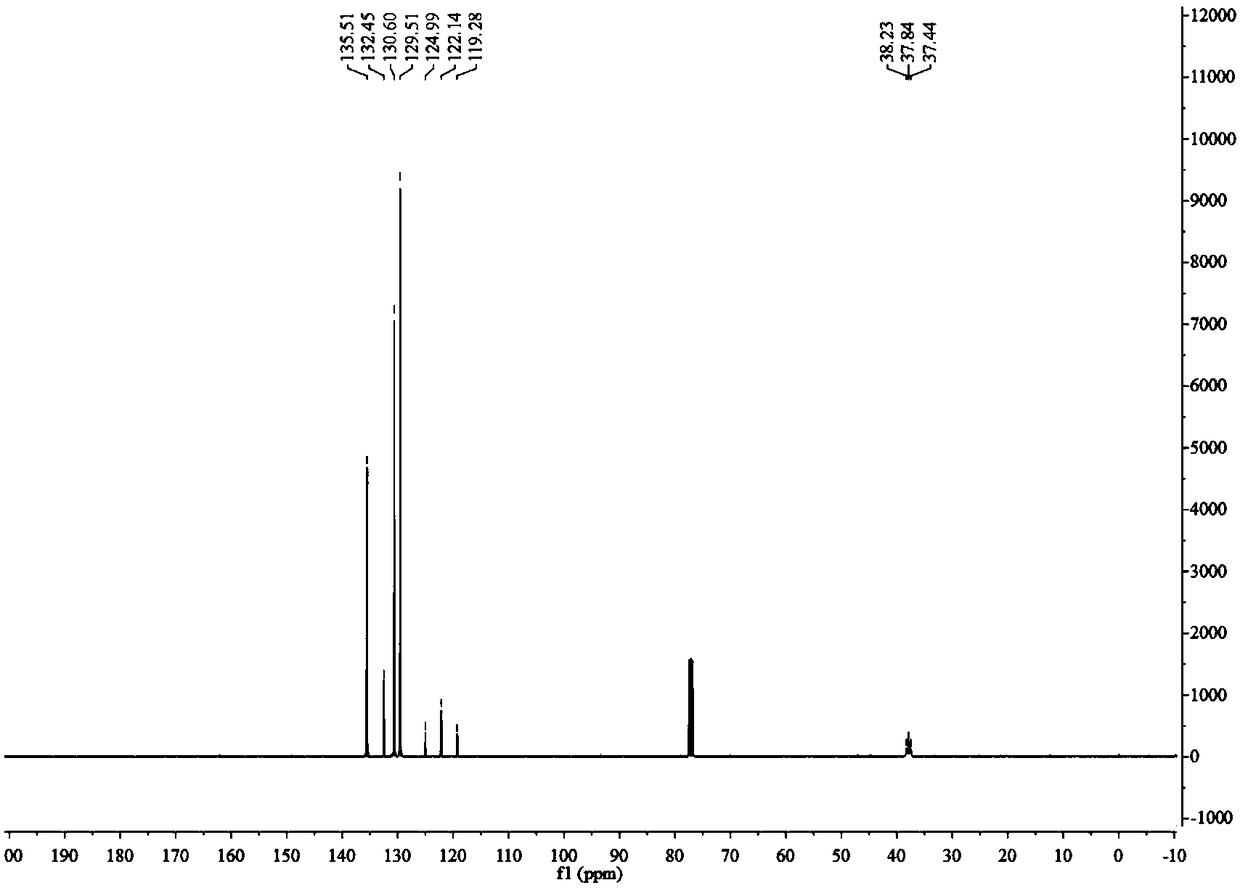
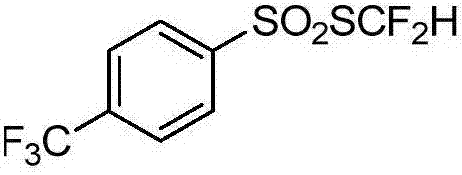
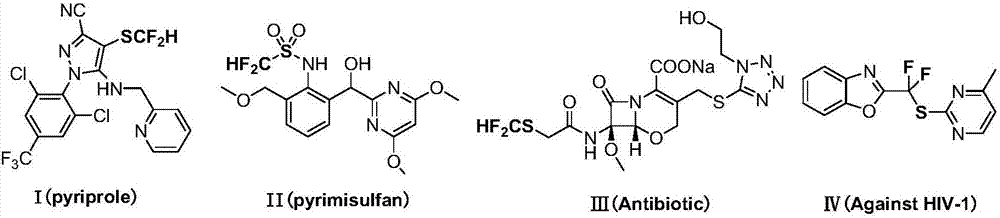
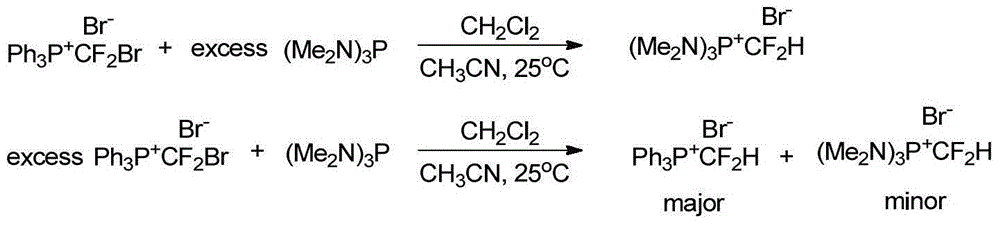
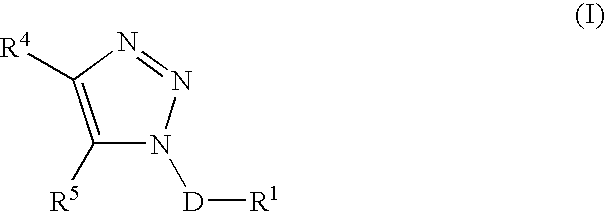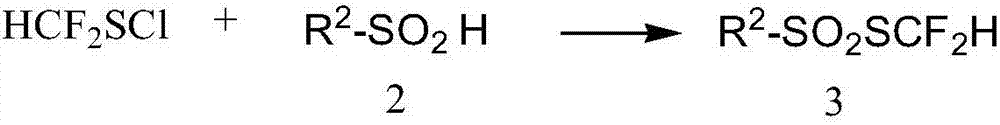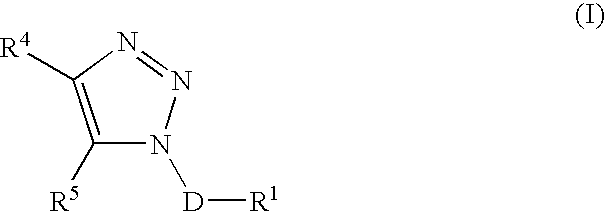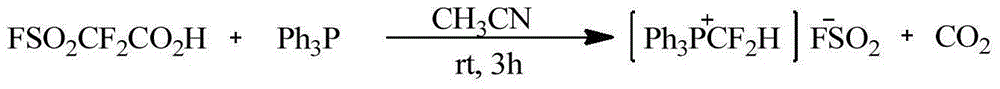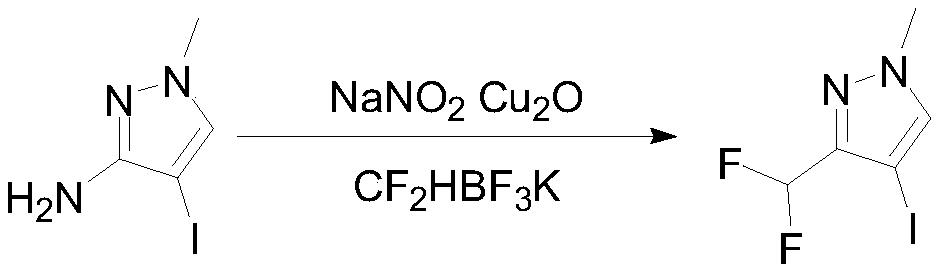Patents
Literature
137 results about "Dichlofopmethyl" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
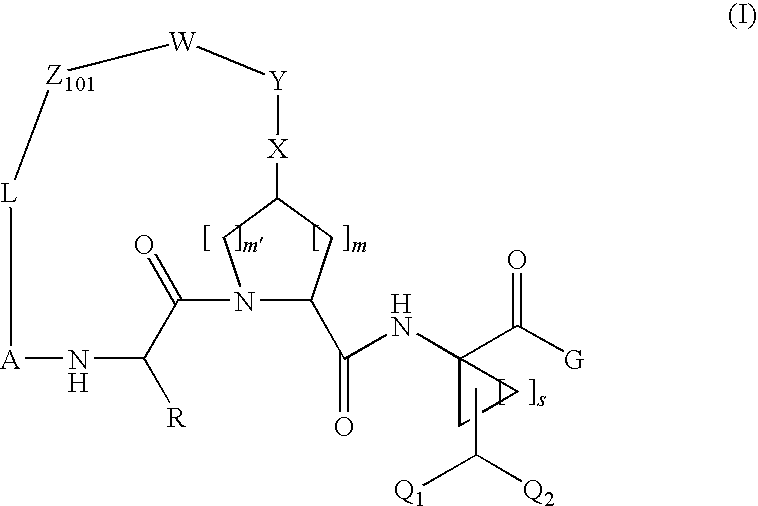
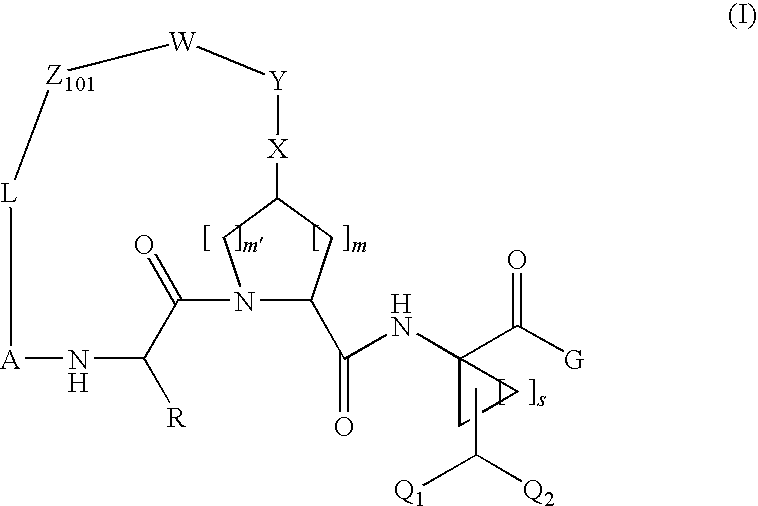
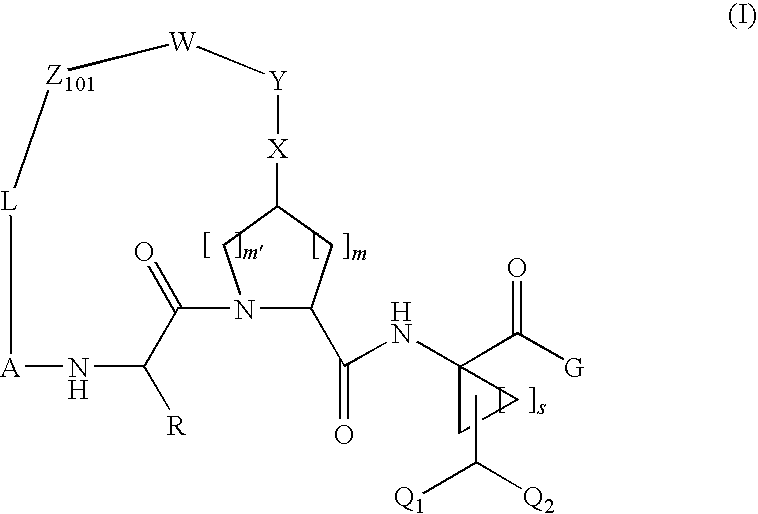
Difluoromethyl-containing macrocyclic compounds as hepatitis c virus inhibitors
The present invention discloses compounds of formula I or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
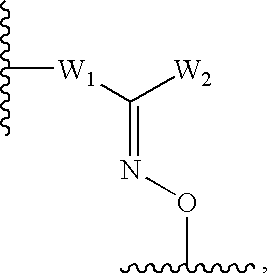
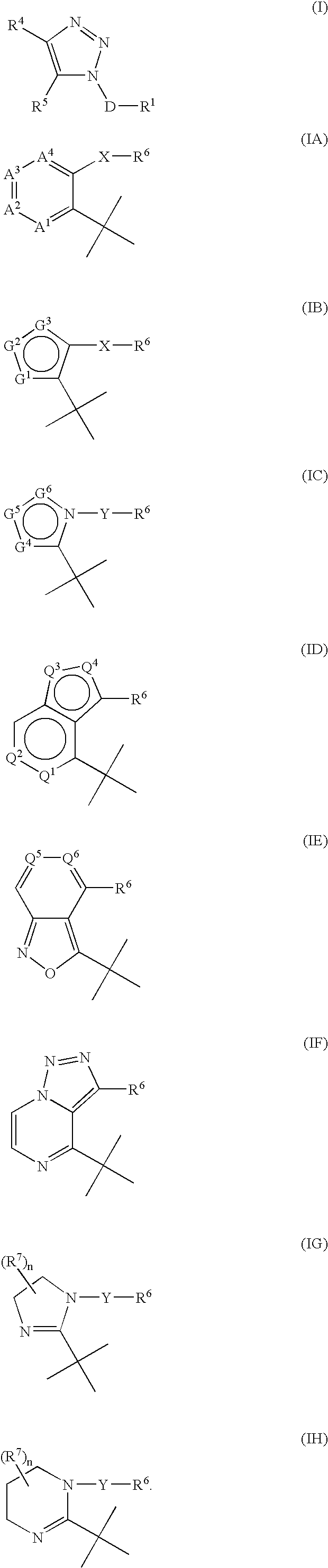
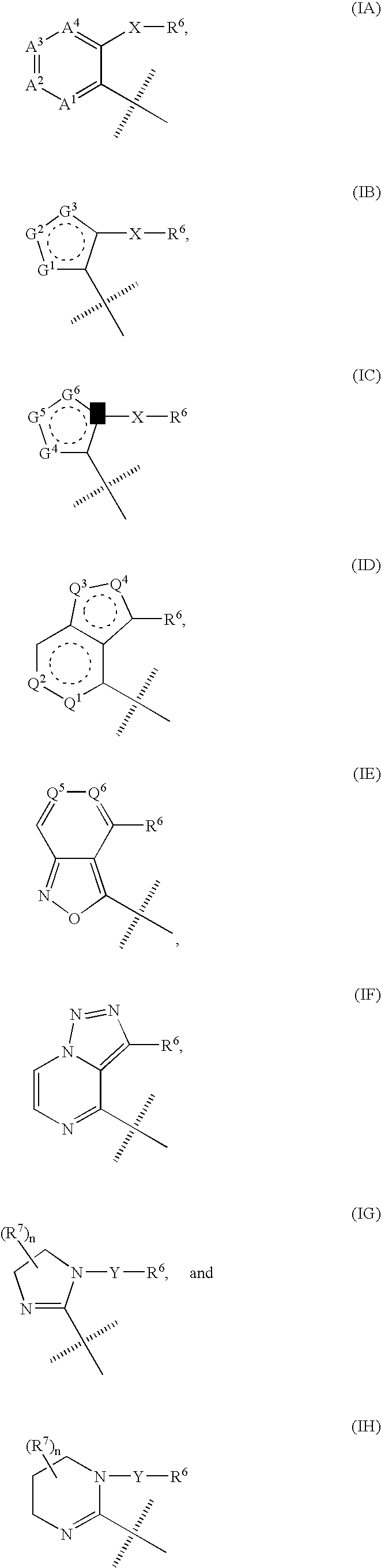
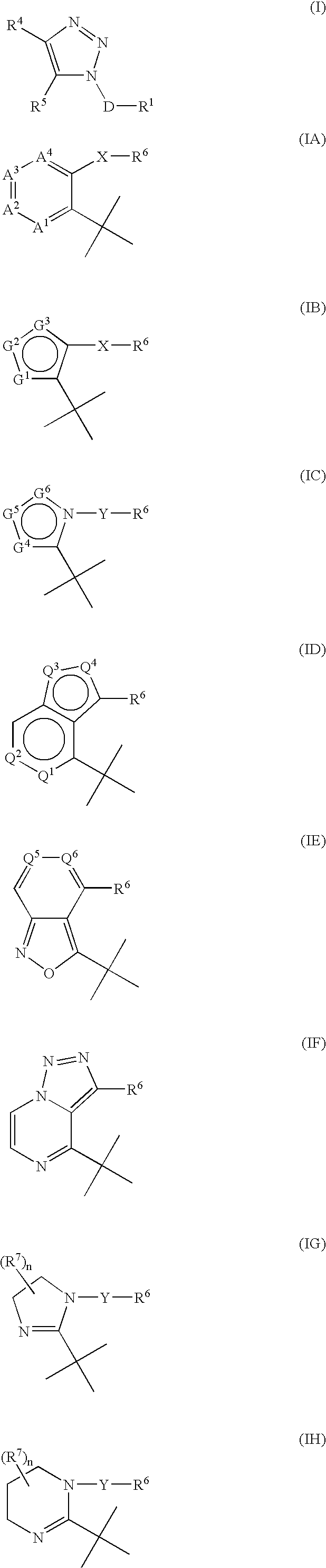
Triazole derivatives as tachykinin receptor antagonists
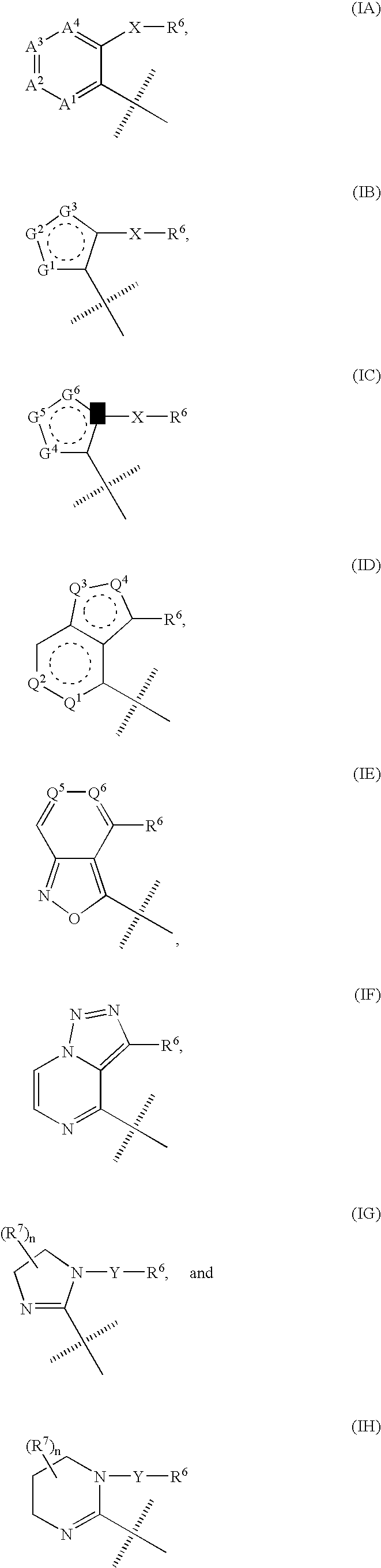
This application relates to a compound of Formula (I) or a pharmaceutically acceptable salt thereof, pharmaceutical compositions thereof, and its use as an inhibitor of the NK-1 subtype of tachykinin receptors, as well as a process for its preparation and intermediates therefor. (I) wherein: D is a C1-C3 alkane-diyl; R1 is phenyl, which is optionally substituted with one to three substitutents independently selected from the group consisting of halo, C1-C4 alkyl, C1-C4 alkoxy, cyano, difluoromethyl, trifluoromethyl, and trifluoromethoxy; R4 is a radical selected from the group consisting of: (IA), (IB), (IC), (ID), (IE), (IF), (IG), (IH)
Owner:ELI LILLY & CO
1-Ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane refrigerant compositions comprising functionalized organic compounds and uses thereof
The present invention relates to compositions for use in refrigeration and air-conditioning systems comprising 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane and at least one chlorocarbon, alcohol, ketone, ether, ester, N-(difluoromethyl)-N,N-dimethylamine, or mixtures thereof. Further, the present invention relates to compositions for use in refrigeration and air-conditioning systems employing a centrifugal compressor comprising 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane and at least one chlorocarbon, alcohol, ketone, ether, ester, N-(difluoromethyl)-N,N-dimethylamine, or mixtures thereof. The compositions of the present invention may be azeotropic or near-azeotropic and are useful in processes for producing cooling or heat or as heat transfer fluids.
Owner:EI DU PONT DE NEMOURS & CO
Fluoroalkyl-substituted derivatives of pyridine, pyrimidine, and pyrazine
Fluoroalkyl-substituted, nitrogen-containing, aryl heterocyclic compounds are provided. The compounds are derivatives of pyridine, pyrimidine, or pyrazine, and have two or three functional groups bonded to the heterocyclic ring, including a perfluoroethyl, perfluoropropyl, perfluoroisopropyl, perfluorobutyl, or difluoromethyl group. Methods of making the compounds using a copper reagent are also provided.
Owner:CATYLIX
2-arylsulfonyl-2, 2-difluorodiazoethane compound, preparation method and application thereof
InactiveCN108383761AEasy to manufactureEasy to useOrganic chemistryOrganic compound preparationCycloadditionAlkyne
The invention relates to a 2-arylsulfonyl-2, 2-difluorodiazoethane compound, a preparation method and application thereof. The preparation method of the 2-arylsulfonyl-2, 2-difluorodiazoethane compound includes: dissolving 2, 2-difluoro-2-aryl sulfonyl ethylamine hydrochloride in a mixed solvent of an organic solvent and water, adding nitrite, carrying out stirring at room temperature for completereaction, and then conducting washing, extraction, separation and column chromatography purification to obtain the yellow liquid 2-arylsulfonyl-2, 2-difluorodiazoethane. The obtained target compoundis a diazo compound containing sulfonyl difluoromethyl, can be subjected to [3+2] cycloaddition reaction with alkyne so as to synthesize a pyrazole compound containing difluoromethyl. The difluorodiazoethane compound provided by the invention has the characteristics of convenient preparation, simple use and mild reaction conditions, and the invention provides an effective method for synthesis of compounds containing difluoromethyl.
Owner:TIANJIN UNIV
Preparation method for difluoromethyl-substituted sulfoaryl sulfonate
The invention discloses a preparation method for difluoromethyl-substituted sulfoaryl sulfonate. The preparation method comprises a step of subjecting a compound as show in a formula 2 which is described in the specification or a salt thereof and difluoromethanesulfonyl chloride to difluoromethylthiolation in an organic solvent so as to obtain a compound as show in a formula 3 which is described in the specification, wherein the organic solvent is one or more selected from a group consisting of a chloralkane organic solvent, a chlorinated aromatic hydrocarbon organic solvent, an aromatic hydrocarbon organic solvent and a nitrile organic solvent . The preparation method of the invention has the advantages of a wide scope of applicable substrates, mild reaction conditions, a high reaction conversion rate, high yield, high product purity and good industrial production prospects.
Owner:SHANGHAI INST OF ORGANIC CHEMISTRY - CHINESE ACAD OF SCI
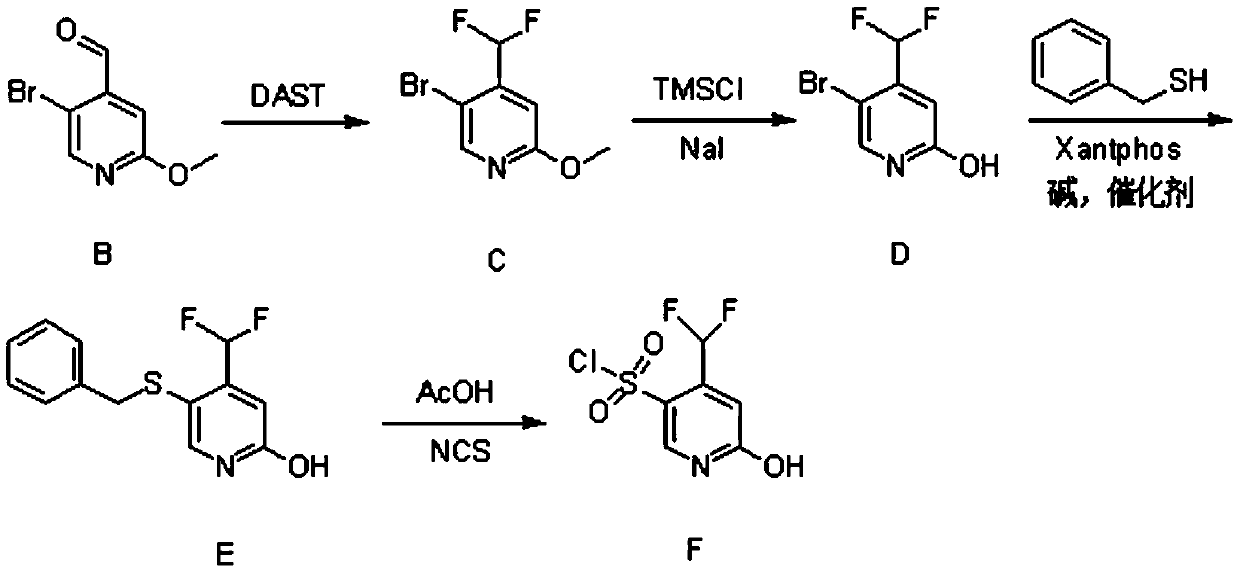
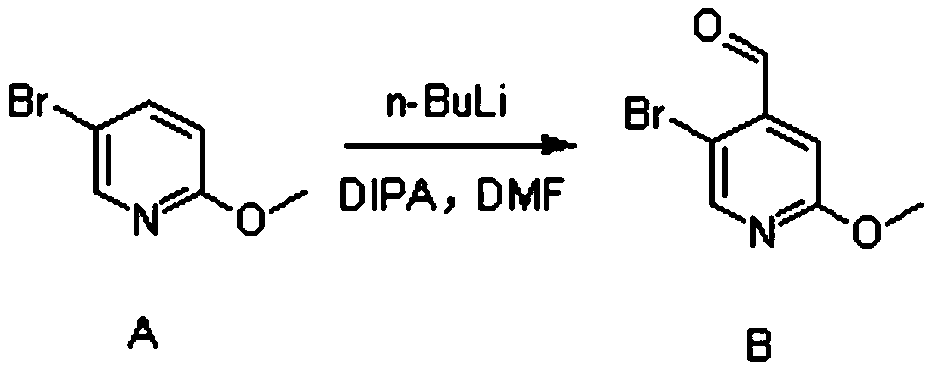
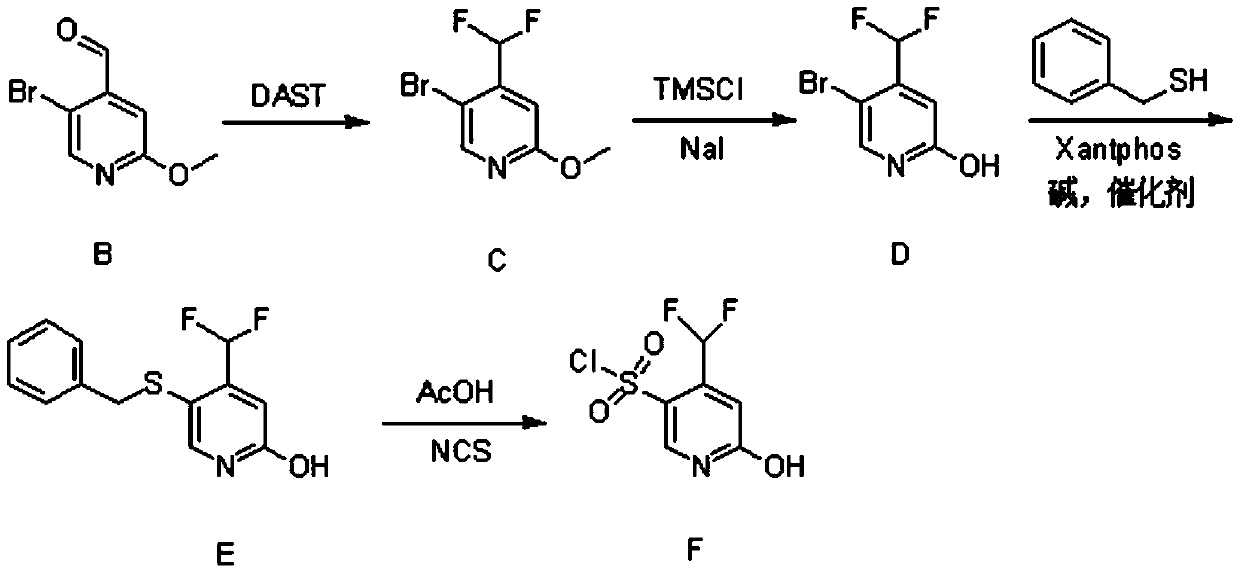
Synthesis method of 4-(difluoromethyl)-2-hydroxypyridine-5-sulfonyl chloride
ActiveCN110734397AHigh selectivityMild reaction conditionsOrganic chemistryOrganic chemicalsDichlofopmethyl
The invention provides a synthetic method of 4-(difluoromethyl)-2-hydroxypyridine-5-sulfonyl chloride, and belongs to the field of organic chemical synthesis. The synthetic method mainly comprises thefollowing steps: reacting a compound B with diethylamino sulfur trifluoride to obtain a compound C; reacting the compound C with sodium iodide and trimethylchlorosilane to obtain a compound D; reacting the compound D with benzyl mercaptan and 4, 5-diphenylphosphine-9, 9-dimethyl xanthene under the action of an alkali and a catalyst to obtain a compound E; and reacting the compound E with N-chlorosuccinimide to obtain a final product compound F. The method is high in selectivity, mild in reaction condition, simple in synthesis operation and easy to implement. In the synthesis process, the compound B is a self-made synthetic material, 5-bromo-2-methoxypyridine is used as a raw material, and nucleophilic substitution reaction is performed to obtain the compound B.
Owner:埃法姆(常州)生命科学技术有限公司
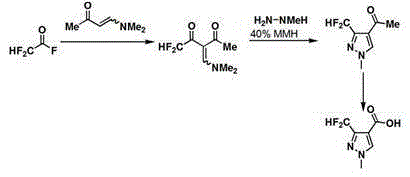
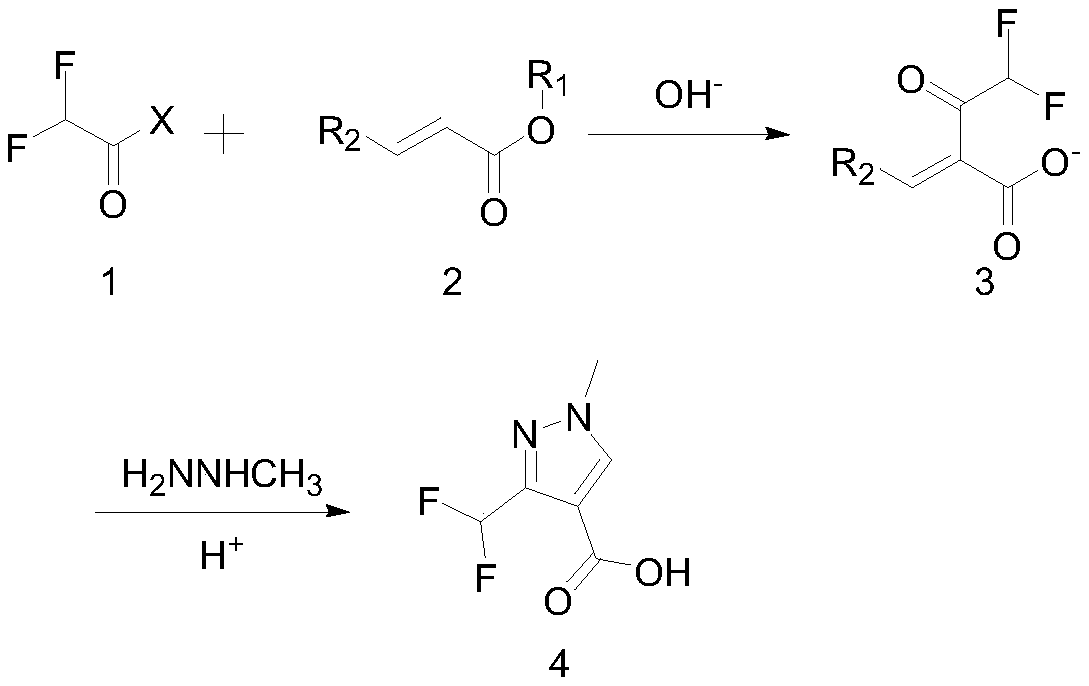
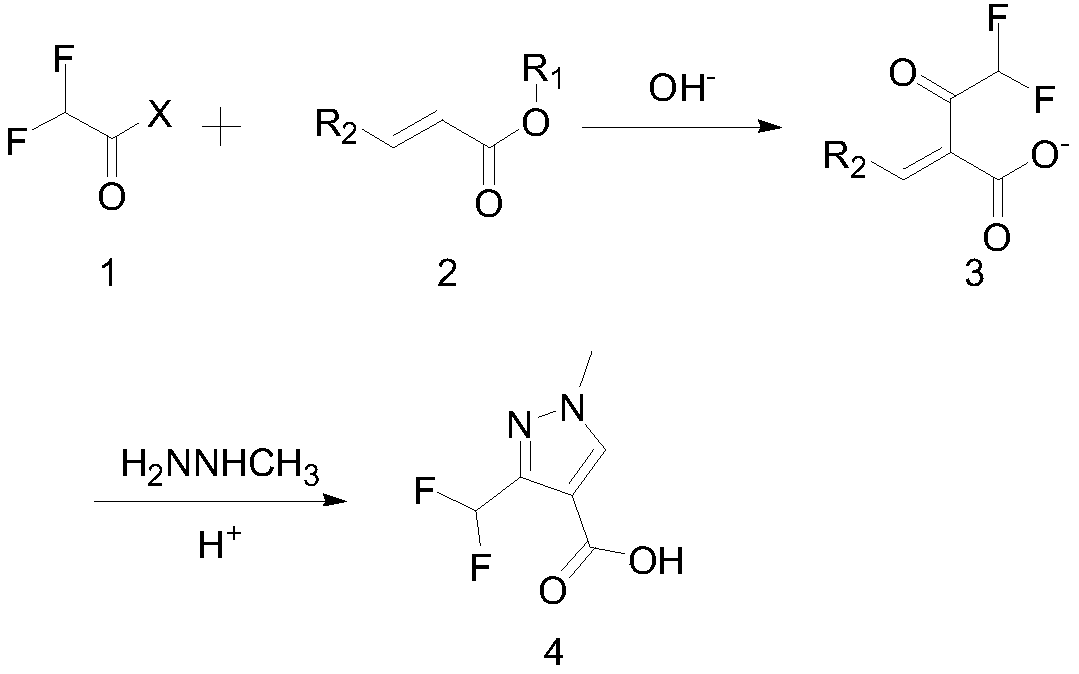
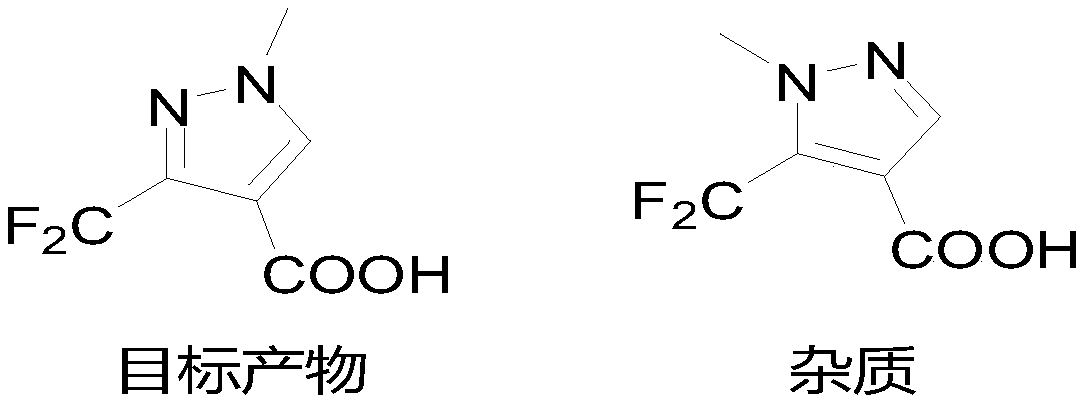
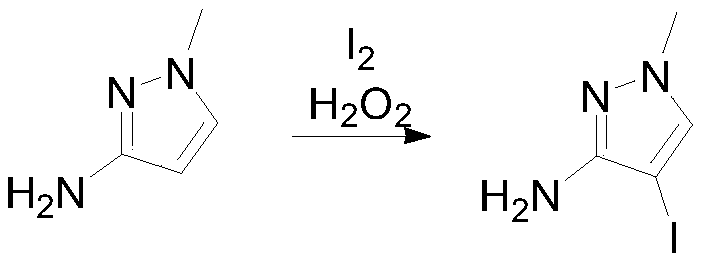
Synthesis method of 3-difluoromethyl-1-methylpyrazole-4-formic acid
InactiveCN106554310ARaw materials are easy to getHigh yieldOrganic chemistrySynthesis methodsHydrazine compound
The invention discloses a synthesis method of 3-difluoromethyl-1-methylpyrazole-4-formic acid, and belongs to the technical field of difluoromethyl pyrazole synthesis. According to the synthesis method, 3-difluoromethyl-1-methylpyrazole-4-formic acid is prepared from 1-dimethylamino-1-butylene-3-one, difluoroacetyl fluoride, and methyl hydrazine through a series of reactions. The provided synthesis method has the advantages of easily available raw materials and high yield, can be applied to industry, and has a comprehensive yield more than 65%.
Owner:陈旭
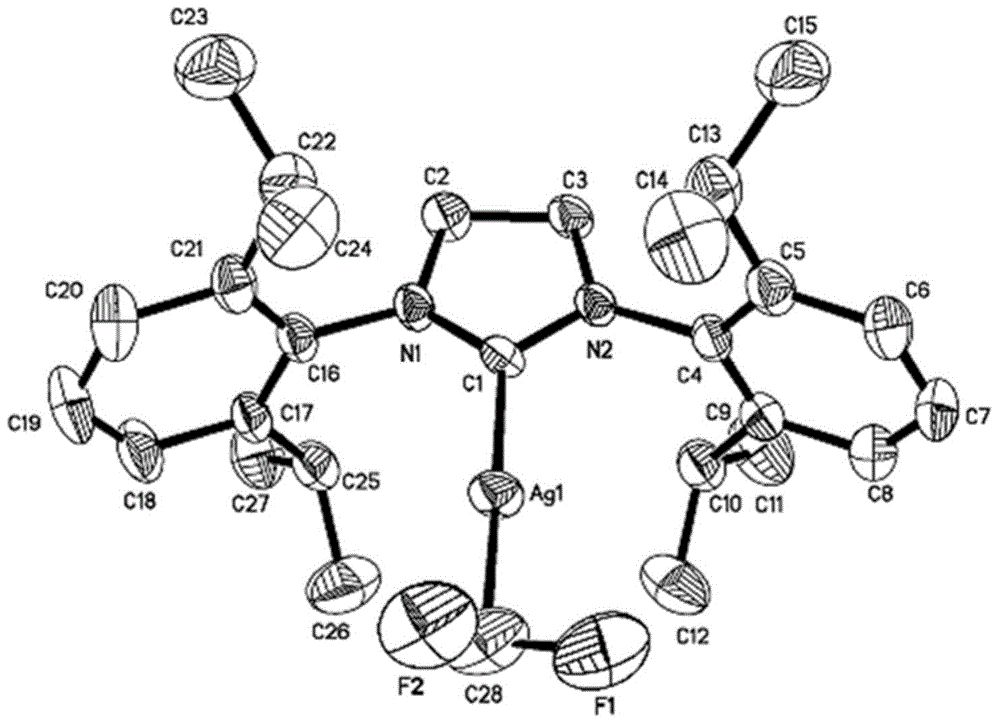
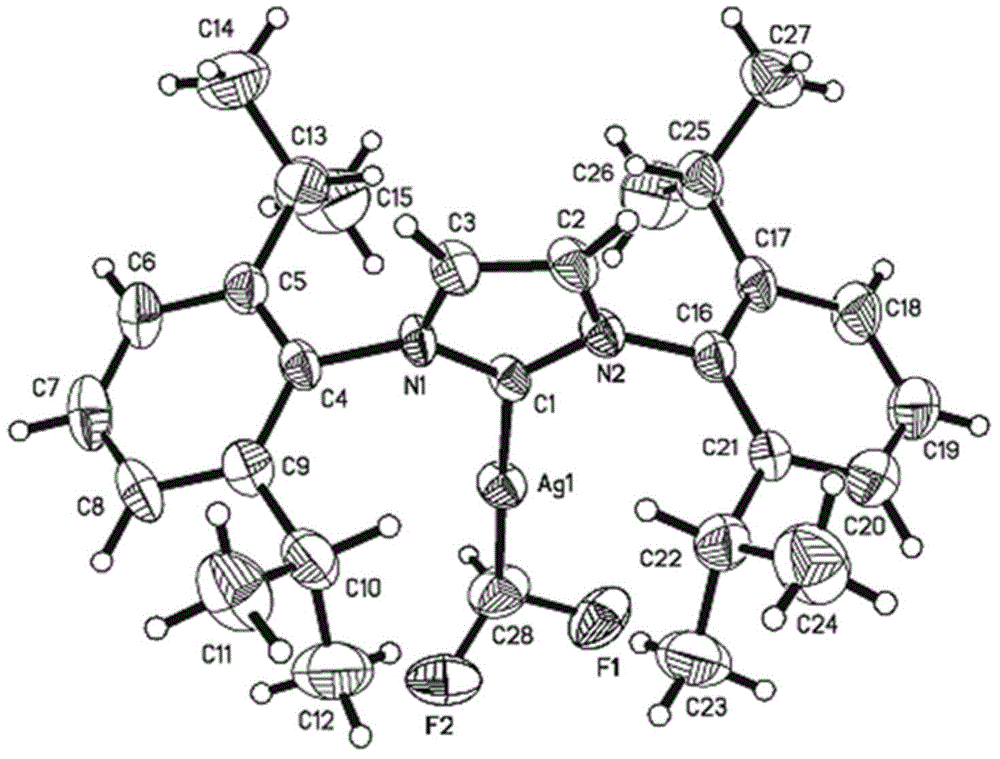
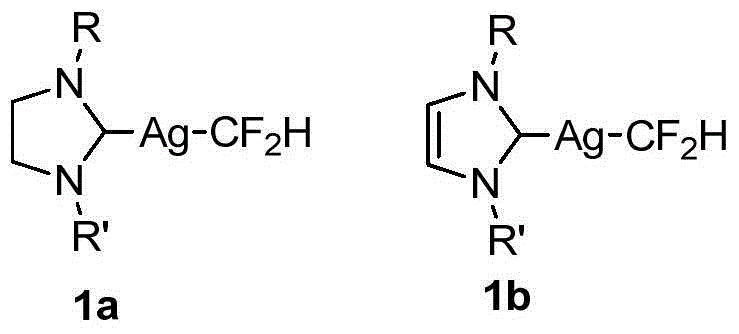
Difluoromethyl silver compound, single crystal, synthetic method and application
ActiveCN104650120AImprove stabilityImprove compatibilityPolycrystalline material growthFrom normal temperature solutionsCarbon numberSingle crystal
The invention discloses a difluoromethyl silver compound, a single crystal, a synthetic method and an application. The invention provides a difluoromethyl silver compound (1a) or a difluoromethyl silver compound (1b), wherein R and R' are independently a phenyl group or a naphthyl group having substituent groups at any position of the phenyl group or the naphthyl group. The substituent group is one or more in number and is an alkyl group with the carbon number of 1-6, an alkoxy group with carbon number of 1-6 or an alkyl-substituted amino group with the carbon number of 1-6, wherein the substituent groups on the phenyl group or the naphthyl group are same or different and the R and R' are same or different. The invention also provides the synthetic method of the difluoromethyl silver compound (1a) or the difluoromethyl silver compound (1b), which includes following steps: carrying out a reaction with aza-carbene complexed silver chloride and trimethyl silyl difluoromethane. The difluoromethyl silver compound is good in stability, can be subjected to an electrophilic substrate through a difluromethylated reaction, is mild in reaction condition, is good in group compatibility and is excellent in market development prospect.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
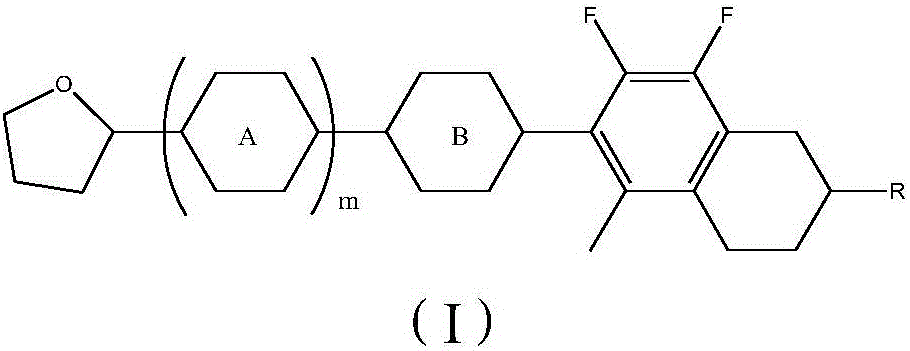
7,8-difluoro-5-methyl-1,2,3,4-tetrahydronaphthalene liquid crystal compounds as well as preparation method and application thereof
ActiveCN106479514AHigh negative dielectric anisotropyLow rotational viscosityLiquid crystal compositionsNon-linear opticsDielectric anisotropyPhenylene
The invention relates to the field of liquid crystal materials, in particular to 7,8-difluoro-5-methyl-1,2,3,4-tetrahydronaphthalene liquid crystal compounds. The compounds have a structure represented as a general formula I, wherein R represents alkyl or alkoxy with 1-12 carbon atoms; a ring A represents 1,4-phenylene, 1,4-cyclohexylidene or 1,4-phenylene with fluorine atoms substituting 1-4 hydrogen atoms; a ring B represents 1,4-phenylene, 1,4-cyclohexylidene, 1,4-cyclohexenylene or 1,4-phenylene with fluorine atoms substituting 1-4 hydrogen atoms; m is 0, 1 or 2. The liquid crystal compounds have the advantages of higher negative dielectric anisotropy, good liquid crystal intersolubility, relatively low rotary viscosity and the like which are needed for improvement of the crystal liquid materials, and the liquid crystal compounds have great application value.
Owner:北京云基科技股份有限公司
N-difluoromethyl hydrazone compound and synthesis method thereof
ActiveCN108794357ANo participationRaw materials are easy to getSulfonic acid amide preparationHydrazone preparationChemical synthesisHydrazone
The invention discloses an N-difluoromethyl hydrazone compound and a synthesis method thereof and belongs to the technical field of chemical synthesis of medicines. The synthesis method comprises thefollowing steps: putting a hydrazone compound, bromine difluoromethyl trimethyl silane, a phase transfer catalyst, an alkali and an organic solvent, heating to 25-110 DEG C, stirring to carry out reactions, and after the reactions are completed, cooling to the room temperature, and separating and purifying products, thereby obtaining the N-difluoromethyl hydrazone compound. The synthesis method disclosed by the invention is easy in raw material obtaining, low in cost, simple and safe to operate, free of metal, good in environment protection, high in functional group adaptability, high in substrate adaptability and good in industrial application prospect.
Owner:SOUTH CHINA UNIV OF TECH
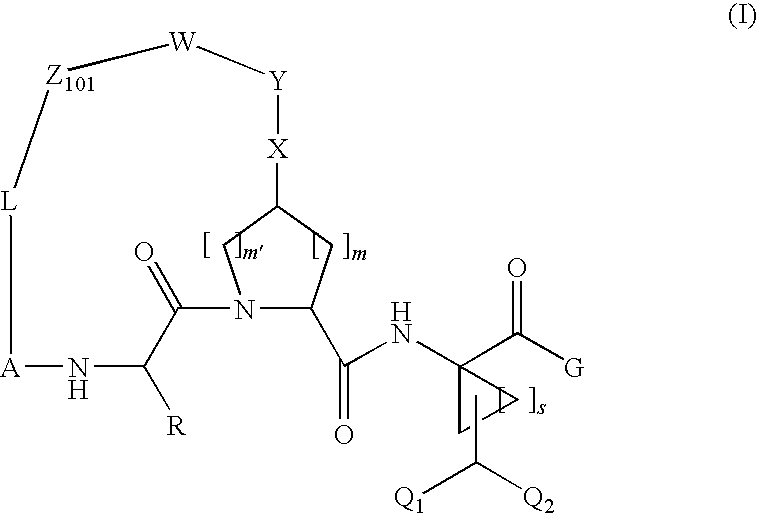
Difluoromethyl-containing macrocyclic compounds as hepatitis C virus inhibitors
The present invention discloses compounds of formula I or pharmaceutically acceptable salts, esters, or prodrugs thereof:which inhibit serine protease activity, particularly the activity of hepatitis C virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
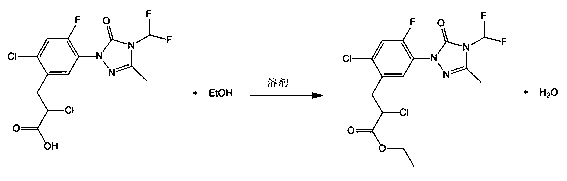
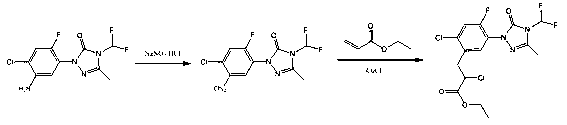
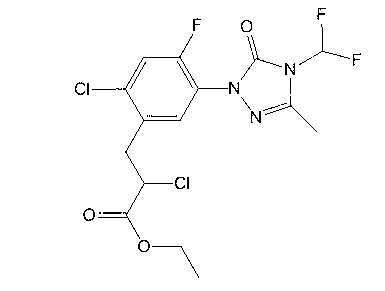
Recovery method of carfentrazone-ethyl
InactiveCN103159688AHigh recovery rateThe new recycling process is simple and efficientOrganic chemistryPropanoic acidProcess engineering
The invention discloses a recovery method of carfentrazone-ethyl. The recovery method is characterized in that the carfentrazone-ethyl in low-boiling wastes is hydrolyzed under an alkaline condition so that high-purity 2-chloro-3-[2-chloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxy-1H-1,2,4-triazole-1-yl]-4-fluorophenyl] propionic acid is obtained; and then the high-purity 2-chloro-3-[2-chloro-5-[4-(difluoromethyl)-4,5-dihydro-3-methyl-5-oxy-1H-1,2,4-triazole-1-yl]-4-fluorophenyl] propionic acid is esterified so that the carfentrazone-ethyl is obtained; the recovery rate reaches 92% and the content of the product is more than 96%. The new recover process provided by the invention is simple and efficient, safe for operation, high in recovery rate and suitable for industrial production.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Triazole derivatives as tachykinin receptor antagonists
This application relates to a compound of Formula (I) or a pharmaceutically acceptable salt thereof, pharmaceutical compositions thereof, and its use as an inhibitor of the NK-1 subtype of tachykinin receptors, as well as a process for its preparation and intermediates therefor. (I) wherein: D is a C1-C3 alkane-diyl; R1 is phenyl, which is optionally substituted with one to three substitutents independently selected from the group consisting of halo, C1-C4 alkyl, C1-C4 alkoxy, cyano, difluoromethyl, trifluoromethyl, and trifluoromethoxy; R4 is a radical selected from the group consisting of: (IA), (IB), (IC), (ID), (IE), (IF), (IG), (IH)
Owner:ELI LILLY & CO
Polymer, resist composition and patterning process
ActiveUS7666571B2Increase contrastDecreased pattern collapsePhotosensitive materialsOriginals for photomechanical treatmentResistPolymer science
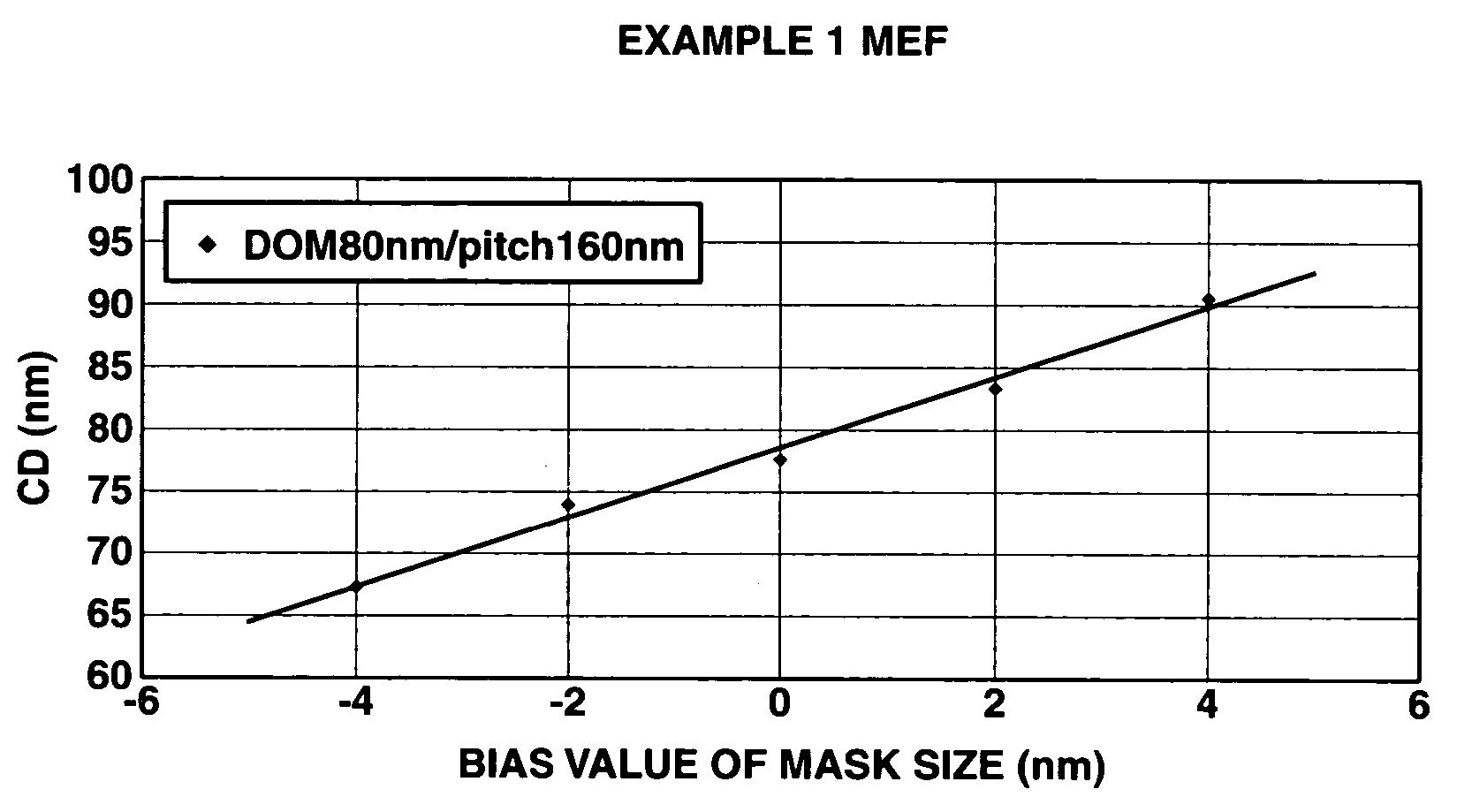
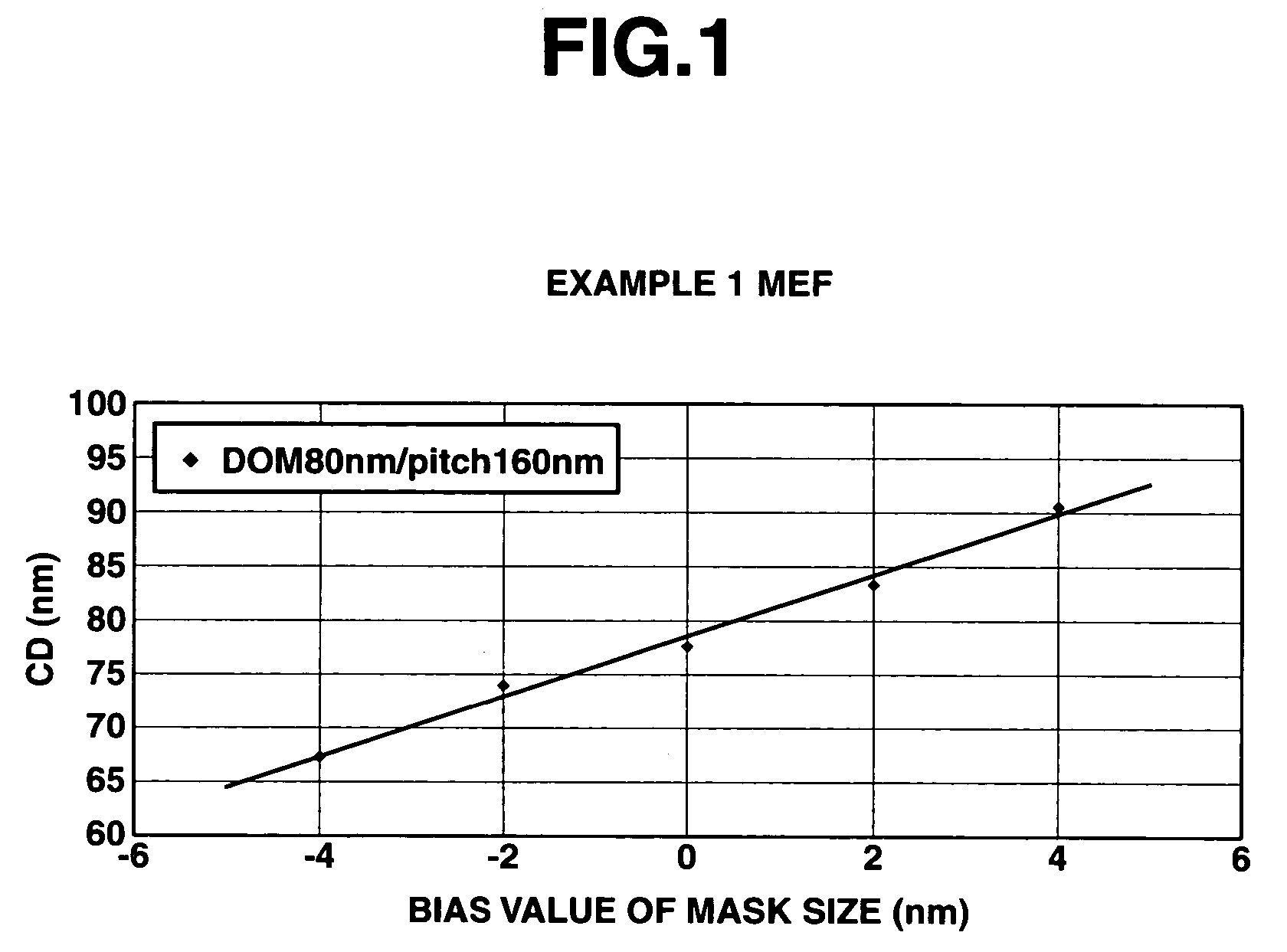
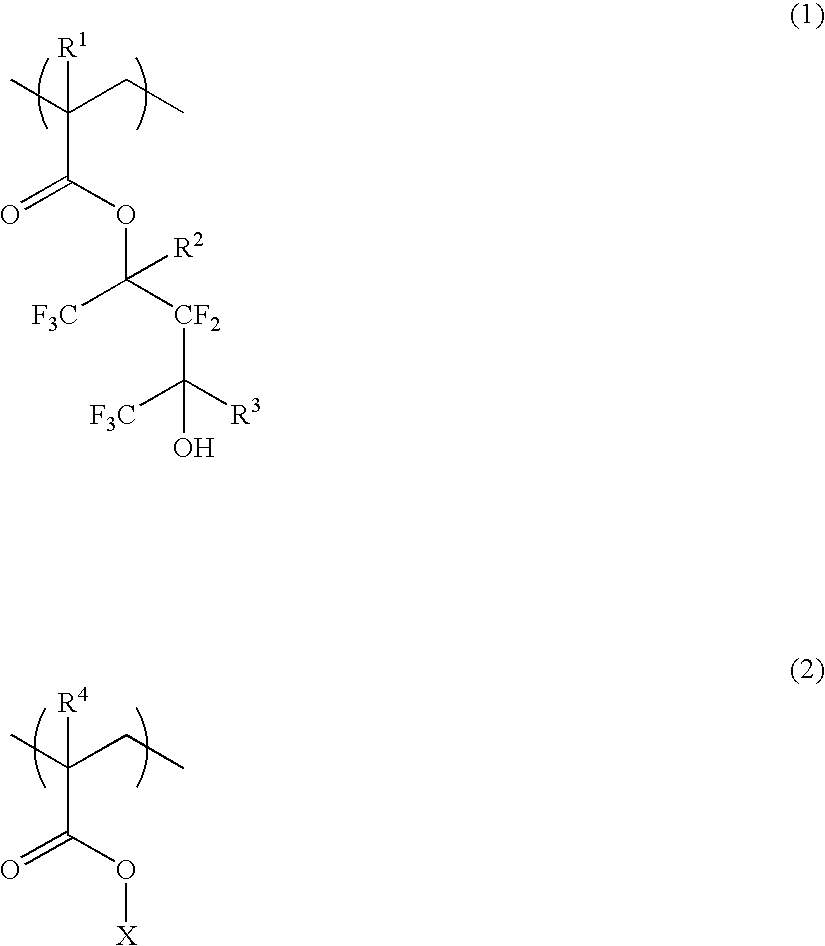
A polymer of which dissolution rate in an alkaline developer increases under the action of acid comprises recurring units having formulae (1) and (2) wherein R1, R2, and R4 are H or methyl, R3 is difluoromethyl or trifluoromethyl, and X is tertiary alkyl. A resist composition comprising the polymer has a high sensitivity and resolution, decreased pattern collapse during development, and minimized MEF and is best suited as micropatterning material for the VLSI manufacture.
Owner:SHIN ETSU CHEM IND CO LTD
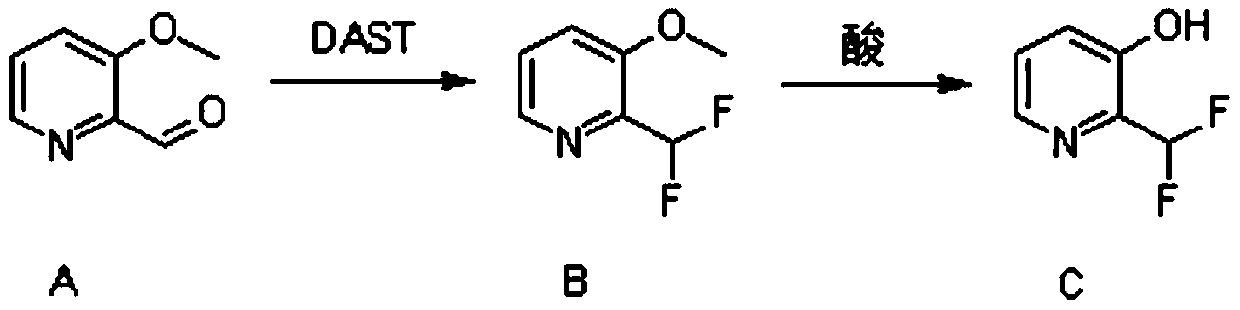
Synthesis method of 2-(difluoromethyl)pyridine-3-ol
ActiveCN110981793AShort process routeReasonable process designOrganic chemistryChemical synthesisOrganic solvent
The invention discloses a synthesis method of 2-(difluoromethyl)pyridine-3-ol, and belongs to the field of organic chemical synthesis. The method comprises the following steps: dissolving 3-methoxypyridine-2-formaldehyde into an organic solvent, and performing reaction with diethylamino sulfur trifluoride under the protection of nitrogen to obtain 2-(difluoromethyl)-3-methoxypyridine; and reactingthe 2-(difluoromethyl)-3-methoxypyridine with an acid to obtain the target product 2-(difluoromethyl)pyridine-3-ol. The method is reasonable in process design, short in route, simple in synthesis operation and easy to implement.
Owner:阿里生物新材料(常州)有限公司
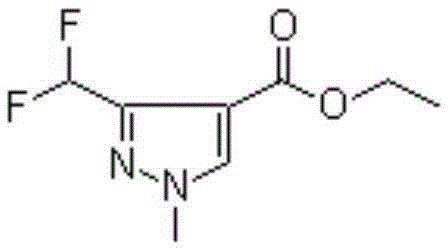
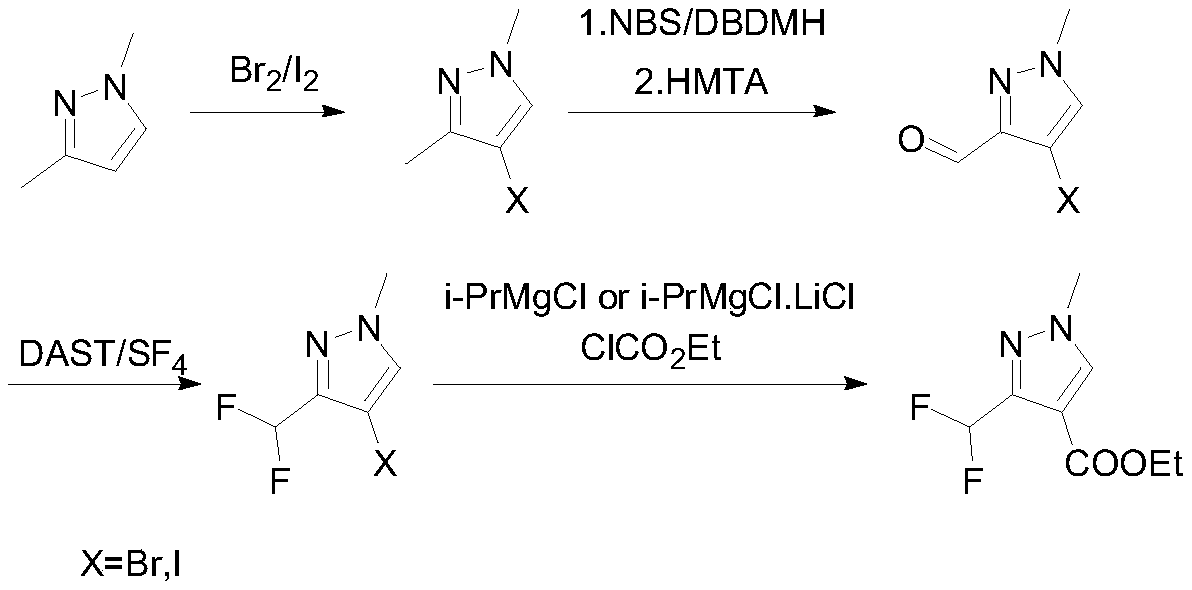
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester
ActiveCN111233768AAvoid it happening againSimple methodOrganic chemistryHexamethylenetetramineOrganic synthesis
The invention discloses a preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester, and belongs to the technical field of organic synthesis. The preparation method comprises the following steps: with 1,3-dimethylpyrazole as a raw material, carrying out halogenation to obtain 4-halogenated-1,3,5-tetrahydronaphthalene; carrying out a reaction under the conditions of a bromination reagent and AIBN and hydrolysis with hexamethylenetetramine to obtain 4-halogen-1-methyl-1H-pyrazole-3-formaldehyde, then performing a reaction with a fluorination reagent to obtain 4-halogen-3-difluoromethyl-1-methylpyrazole; finally carrying out a reaction on the 4-halogen-3-difluoromethyl-1-methylpyrazole and a Grignard reagent to obtain 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid ethyl ester. The method is simple and convenient to operate and high in reaction yield, the purity of the obtained product can reach 99.5% or above, and the method has a potential process amplification prospect.
Owner:徐州圣元化工有限公司
Difluromethylphosphonium salt, and preparation method and application thereof
InactiveCN106146556AWide applicabilitySimple and fast operationCarboxylic acid nitrile preparationOrganic compound preparationPhosphoniumKetone
The invention discloses difluromethylphosphonium salt, and a preparation method and application thereof. A synthetic method of the difluromethylphosphonium salt disclosed by the invention comprises the following steps of in a solvent, carrying out a reaction shown as follows on gem-difluoromethylenated phosphonium inner salt as shown in a formula II and protonic acid, and preparing a compound as shown in a formula I. The invention discloses application of the compound as shown in the formula I in difluromethylation reaction with a compound as shown in a formula III or a compound as shown in a formula IV. The preparation method of the difluromethylphosphonium salt provided by the invention is simple and convenient to operate and wide in substrate applicability, can be carried out with the existence of water and oxygen, and is moderate in reaction condition and high in yield and purity. The difluromethylphosphonium salt provided by the invention can be used for directly difluromethylating aldehyde, ketone and imine compounds, and the method is simple to operate, moderate in condition, and high in reaction yield when a substrate is a ketone compound containing alpha-H.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Difluoromethylation Preparation method for 2-(2,4-dichlorophenyl)-1,2-dihydrogen-5-methyl-3H-1,2,4-triazole-3-ketone
The invention discloses a difluoromethylation Preparation method for 2-(2,4-dichlorophenyl)-1,2-dihydrogen-5-methyl-3H-1,2,4-triazole-3-ketone. The method comprises the following steps: triadimefon is led to react with potassium carbonate to obtain potassium triadimefon using xylol as reaction solvent and in the presence of phase transfer catalyst; the potassium triadimefon is then let to react with chlorodifluoromethane at the temperature of 150-200 DEG C; and the 2-(2,4-dichlorophenyl)-1,2-dihydrogen-5-methyl-3H-1,2,4-triazole-3-ketone is prepared at a high yield. The method is simple and highly efficient, is safe in operation and high in yield, and facilitates industrial production.
Owner:JIANGSU BAOZONG & BAODA PHARMACHEM
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
ActiveCN111362874ASimple and fast operationRaw materials are easy to getOrganic chemistryPtru catalystOrganic synthesis
The invention discloses a preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid, and belongs to the technical field of organic synthesis. The preparation method comprises thefollowing steps: by taking 2, 2-difluoroacetyl halide as a raw material, performing addition with alpha, beta-unsaturated ester, performing alkaline hydrolysis to obtain alpha-difluoroacetyl intermediate carboxylic acid, condensing and cyclizing the 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid with a methylhydrazine aqueous solution in the presence of a catalyst to obtain a 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid crude product, and recrystallizing to obtain a pure product of the 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid. The method has the advantages of simple operation, easily available raw materials, high reaction yield, reduction of isomers through a plurality of ways in the reaction process, and convenience in product purification.
Owner:徐州圣元化工有限公司
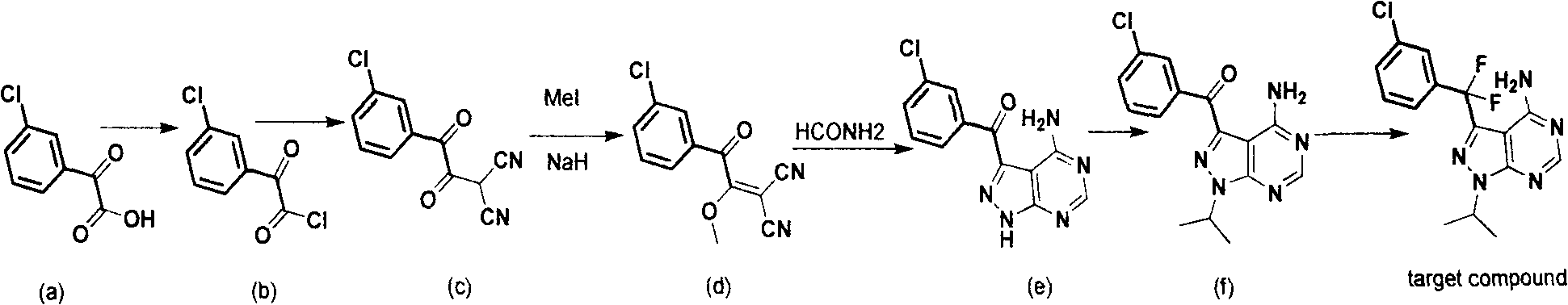
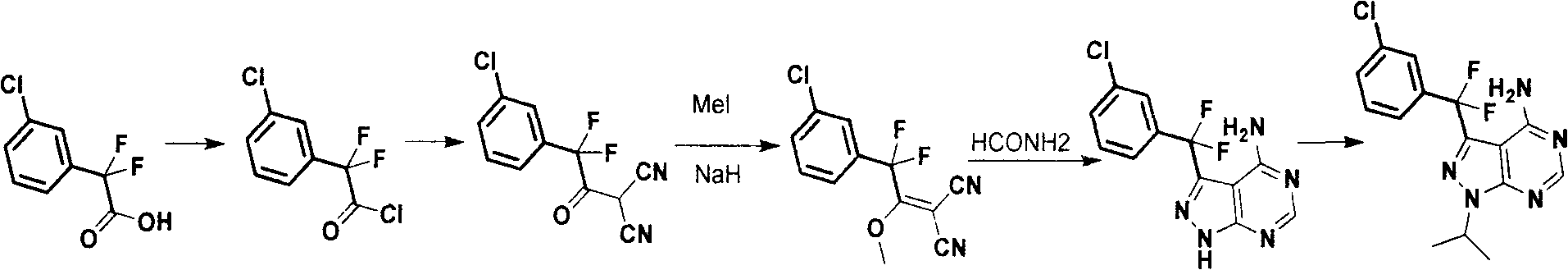
Synthesis process for anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound
The invention discloses a synthesis method for an anti-tumor new medicine 3-{(3-chlorphenyl)difluoromethyl-1-isopropyl-monohydro-pyrazolo(3,4-D)pyrimidine-4-amine compound. The synthesis method comprises the following steps of: acylating 3-chlorphenyl-2 oxalic acid serving as an initiative raw material; reacting the acylated material with dipropionitrile; performing methylation; performing ring closure with hydrazine hydrate to obtain 5-amino-1-(3-chlorophenyl)-pyrazole-4-carbonitrile, crystallizing and purifying, heating and performing ring closure with formamide to obtain a target mother ring; performing fluorination on carbonyl by using DAST; introducing difluorine into an active group so as to improve the bioactivity of the compound; performing process optimization on the basis of the compound; and performing the similar steps by using 2-(3-chlorphenyl)-2,2-difluoroacetic acid as a raw material to obtain the target product.
Owner:CGENETECH (SUZHOU CHINA) CO LTD
Difluoromethyl selenate compound and synthesis method thereof
ActiveCN112279796AImprove toleranceEasy to operateSteroidsBulk chemical productionSelenateMethyl group
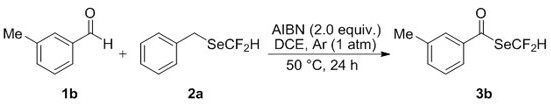
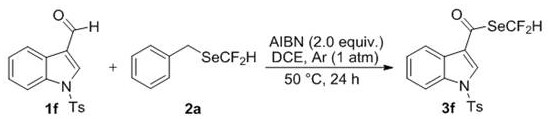
The invention discloses a difluoromethyl selenate compound and a synthesis method thereof. The synthesis method comprises the following step of: by taking a selenium difluoromethylation reagent and cheap and readily available aldehyde or aldehyde group-containing bioactive molecules as initial raw materials, taking an azo compound as a free radical initiator and taking 1, 2-dichloroethane as a solvent, synthesizing the difluoromethyl selenate compound through free radical reactionin an argon atmosphere. The synthesis method is mild in condition, wide in substrate application range and good infunctional group tolerance, particularly has no metal participation, and has the advantages of being easy to operate, environmentally friendly, complete in regioselectivity and the like.
Owner:NORTHWEST UNIV
1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane refrigerant compositions comprising functionalized organic compounds and uses thereof
The present invention relates to compositions for use in refrigeration and air-conditioning systems comprising 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane and at least one chlorocarbon, alcohol, ketone, ether, ester, N-(difluoromethyl)-N,N-dimethylamine, or mixtures thereof. Further, the present invention relates to compositions for use in refrigeration and air-conditioning systems employing a centrifugal compressor comprising 1-ethoxy-1,1,2,2,3,3,4,4,4-nonafluorobutane and at least one chlorocarbon, alcohol, ketone, ether, ester, N-(difluoromethyl)-N,N-dimethylamine, or mixtures thereof. The compositions of the present invention may be azeotropic or near-azeotropic and are useful in processes for producing cooling or heat or as heat transfer fluids.
Owner:EI DU PONT DE NEMOURS & CO
Synthesis method of 1, 1-difluoro-2-isonitrile-ethyl phenyl sulfone compound
ActiveCN110790689ACircumvention of restore stepsHigh selectivityOrganic compound preparationSulfide preparationEthyl groupALUMINUM HYDRIDE
The invention discloses a synthesis method of a 1, 1-difluoro-2-isonitrile-ethyl phenyl sulfone compound. The synthesis method is characterized in that the 1, 1-difluoro-2-isonitrile-ethyl phenyl sulfone compound is synthesized from a 2, 2-difluoro-2-(thiophenyl) ethyl acetate compound through the steps of reduction, oxidation, alcohol amination, formylation, dehydration reaction and the like. According to the method, reducing agent lithium aluminum hydride which is dangerous and poor in selectivity is prevented from being used for reducing a difluoromethyl amide intermediate low in reaction activity; the mild reducing agent sodium borohydride is adopted to reduce the 2, 2-difluoro-2-( thiophenyl) ethyl acetate compound high in activity, so that the reduction yield and selectivity are greatly improved, and the method is simple to operate, stable in product property and beneficial to large-scale production.
Owner:JIANGXI NORMAL UNIV
Preparation and application of difluoromethyl pyrazole compound comprising 1,3,4-oxadiazole structure
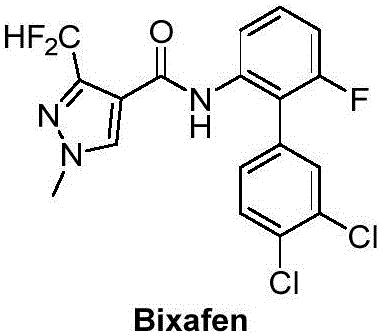
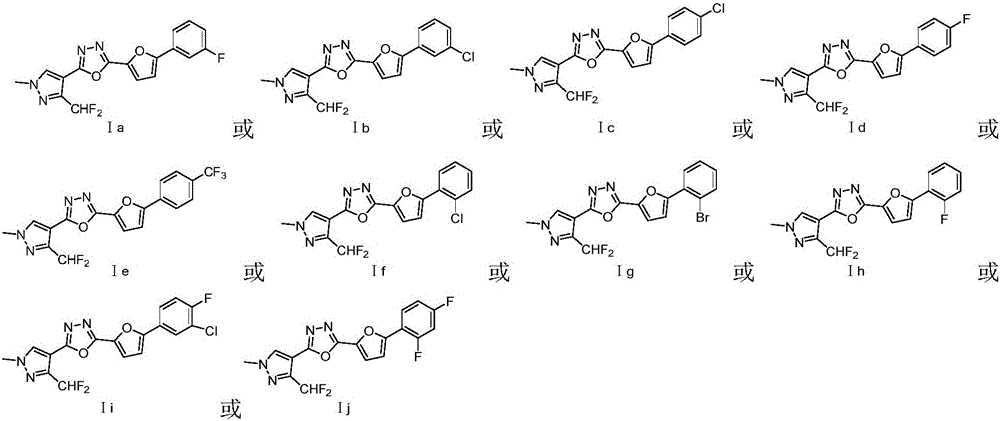
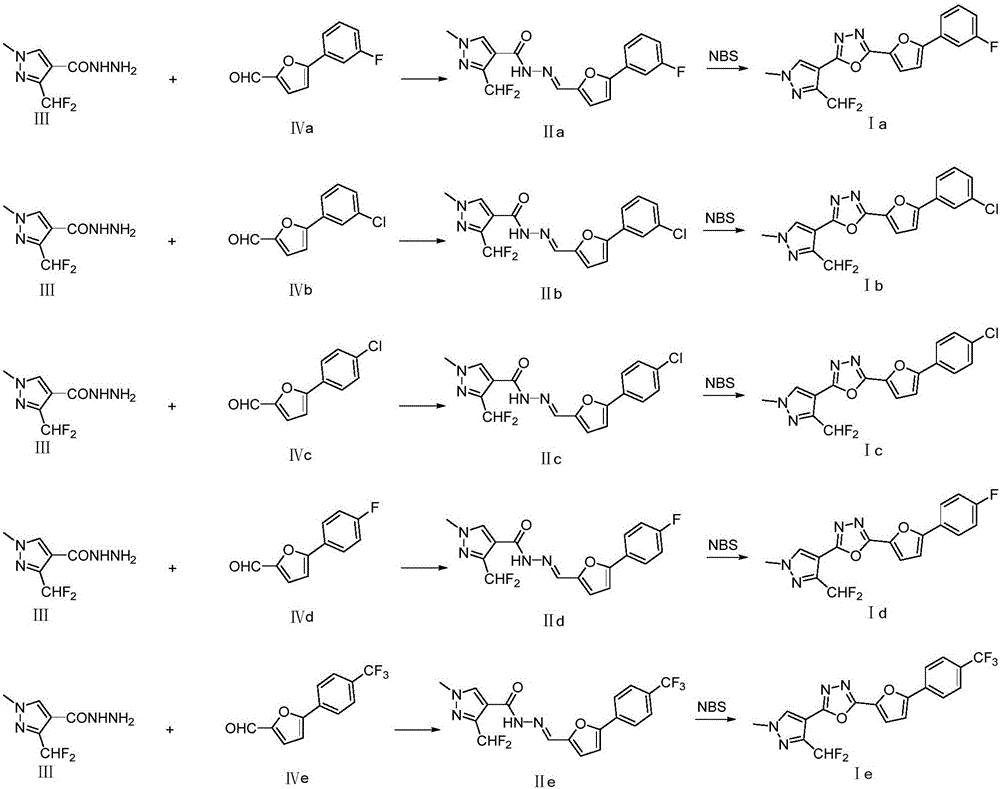
The invention relates to difluoromethyl pyrazole compound (I) comprising 1,3,4-oxadiazole structure and a preparation method and application thereof.The compound is obtained by dehydration and cyclization of pyrazole hydrazine intermediate (II) comprising furan groups.The difluoromethyl pyrazole compound comprising 1,3,4-oxadiazole structure is effective in controlling harmful insects and is useful in the preparation of insecticides for agricultural field, gardening field and other fields.
Owner:安徽先胜达农药有限公司
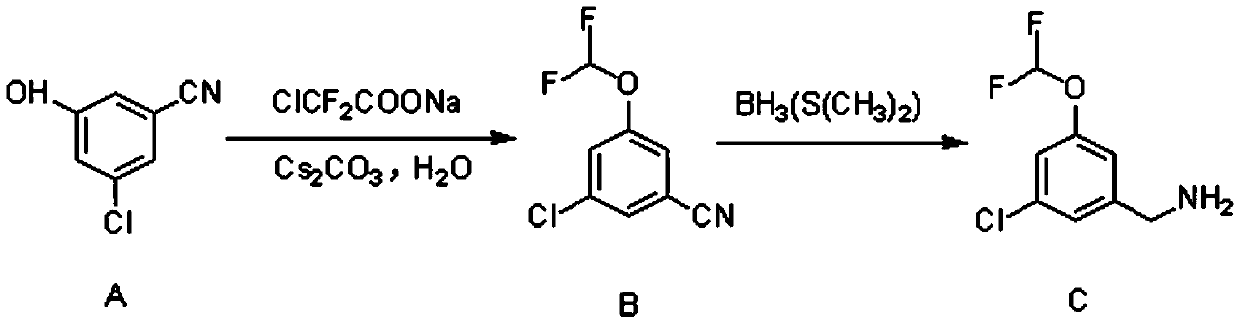
Synthetic method of 3-chloro-5-(difluoromethoxy)benzylamine
ActiveCN110885291AReasonable process designEasy to operateCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisBiochemical engineering
The invention provides a synthetic method of 3-chloro-5-(difluoromethoxy)benzylamine, and belongs to the field of chemical synthesis of medicines. According to the method, 3-chloro-5-hydroxybenzonitrile is used as a raw material, and under the protection of nitrogen, the final product 3-chloro-5-(difluoromethoxy)benzylamine is obtained through difluoromethylation and reduction. The method is reasonable in process design, short in route, simple to operate and easy to control.
Owner:埃法姆(常州)生命科学技术有限公司
Synthesis of difluoromethyl ethers and sulfides
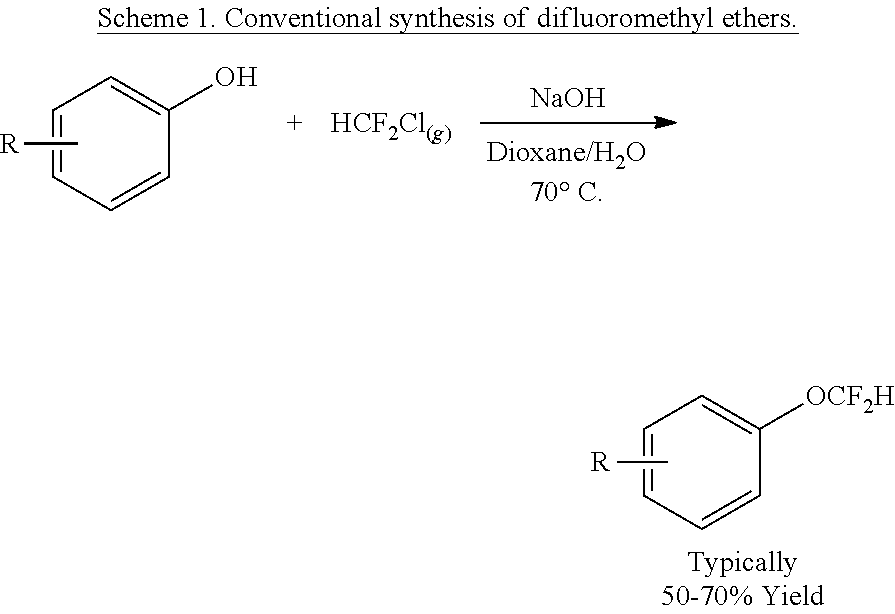
InactiveUS20150336866A1Highly versatileReadily availableCarboxylic acid nitrile preparationOrganic compound preparationArylPHENOL LIQUID
The synthesis of difluoromethyl ethers and sulfides with a simple, non-ozone-depleting reagent is described. The difluoromethylation of phenols with this reagent occurs at room temperature within minutes with exceptional functional group tolerance. The mild conditions makes possible tandem processes for the conversion of aryl boronic acids, aryl halides and arenes to difluoromethyl ethers. Mechanistic studies support a reaction pathway involving nucleophilic attack of the phenolate to difluorocarbene.
Owner:RGT UNIV OF CALIFORNIA
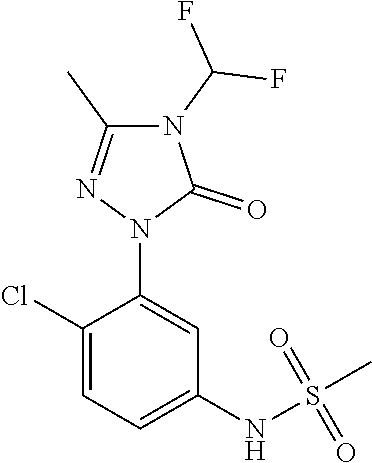
Purification of N-(2,4-dichloro-5-(4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl) methanesulfonamide herbicide using selective pH adjusted extractions
ActiveUS20220106277A1Reduce presenceBiocideOrganic compounds purification/separation/stabilisationMeth-Organic solvent
A high yielding extraction process for the purification of N-(2,4-dichloro-5-(4-(difluoromethyl)-3-methyl-5-oxo-4,5-dihydro-1H-1,2,4-triazol-1-yl)methanesulfonamide (sulfentrazone) by selectively partitioning the desired product from the crude mixture, thereby increasing its purity by decreasing the presence of unwanted impurities and improving the color and particle size distribution of the final sulfentrazone product. The selective partitioning is achieved by the sequential use of an organic solvent, water, aqueous inorganic base and a concentrated aqueous inorganic acid.
Owner:TAGROS CHEM INDIA PVT LTD
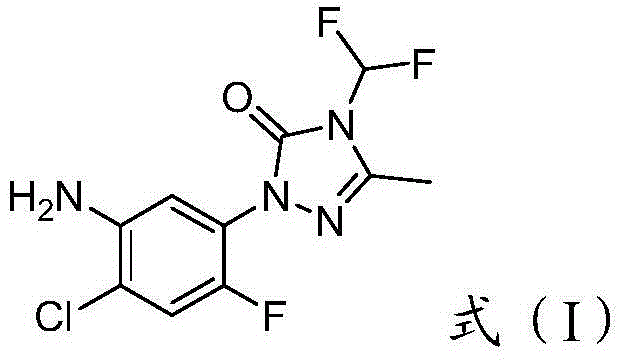
Method for purifying 2-(2-flourine-4-chlorine-5-aminophenyl)-4-difluromethylation-5-methyl-1,2,4-triazole-3-ketone
The invention provides a method for purifying 2-(2-flourine-4-chlorine-5-aminophenyl)-4-difluromethylation-5-methyl-1,2,4-triazole-3-ketone. The method comprises the following steps: firstly, forming an acid from a crude product; decolorizing; and neutralizing to obtain a pure product. The purification process is free of demands on the purity of the crude product; other purifying auxiliary materials are cheap and available; the purifying steps are simple to operate; and industrial production is easy to achieve.
Owner:HEFEI JIUYI AGRI DEV
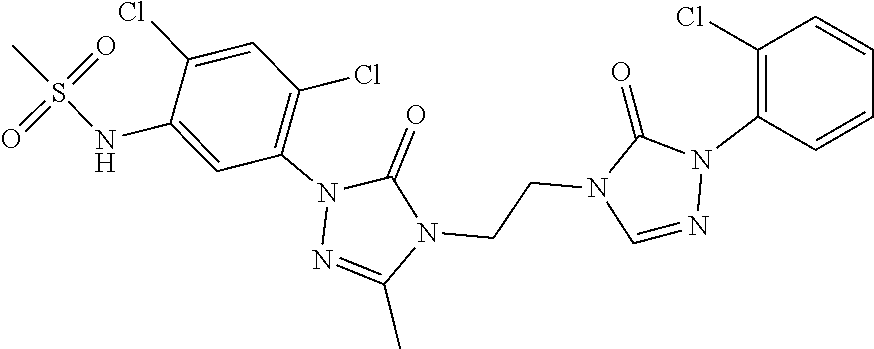
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
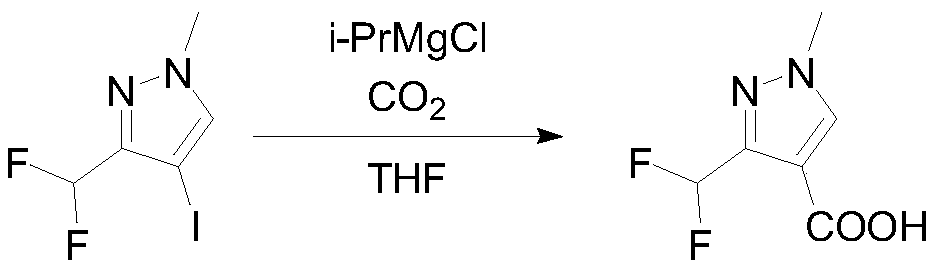
InactiveCN111303035ASimple and fast operationHigh reaction yieldProductsOrganic chemistryIsopropylOrganic synthesis
The invention discloses a preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid, and belongs to the technical field of organic synthesis. The preparation method comprises thefollowing steps: reacting N-methyl-3-aminopyrazole as a raw material with bromine / iodine to replace pyrazole at site 4, then carrying out diazotizing and coupling with potassium difluoromethyl trifluoroborate to obtain 4-bromo-3-(difluoromethyl)-1-methyl-1H-pyrazole, then performing Grignard exchange by adopting isopropyl magnesium chloride and the like, and performing reaction with carbon dioxide to obtain 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid. The method is simple and convenient to operate, the total yield of the three steps is as high as 64%, the product purity can reach 99.5% or above, the situation in which isomers exist in a traditional process is avoided, and the method has potential process amplification prospects.
Owner:徐州圣元化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com