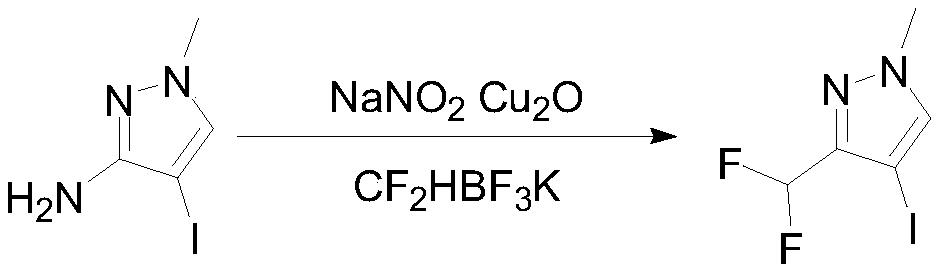Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
A technology of potassium difluoromethyltrifluoroborate and difluoromethyl, which is applied in organic chemistry, products, reagents, etc., can solve the problems of spending a lot of energy and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
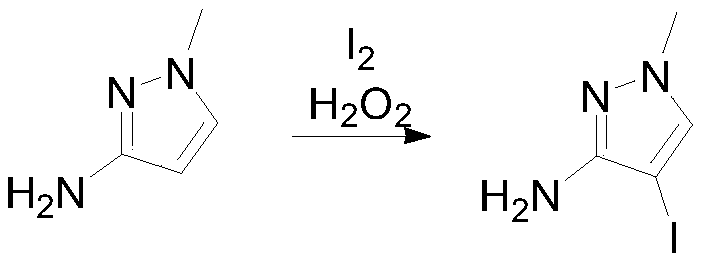
[0025] Into a three-necked reaction flask, put 97.1g (1mol, 1.0eq) of N-methyl-3-aminopyrazole, 800mL of water and 500mL of ethanol, and add 129.5g (0.51mol, 0.51eq) of iodine in batches at a controlled temperature of 15-25°C ), 72.9g (0.6mol, 0.6eq) of 28% hydrogen peroxide was added dropwise at 15-25°C. Raise the temperature to 30-35°C, react for 6 hours, sample GC material 1 HNMR (400MHz, CDCl 3 ):7.40(s,1H),5.81(s,2H),3.80(s,3H).
[0026]
[0027] Put 4-iodo-1-methyl-1H-pyrazol-3-amine (100g, 0.45mol) and 6mol / L hydrochloric acid aqueous solution into the reaction bottle, adjust the pH<1, cool down to -5°C, slowly add 40 % sodium nitrite aqueous solution 78.1g, reacted at -5-5°C for 1 hour after the dropwise addition, and transferred to the dropping funnel under nitrogen protection.
[0028] Under the protection of nitrogen, add 73.3g (470.8mmol, 1.05eq) of potassium difluoromethyltrifluoroborate, 4.5g (31.4mmol, 0.07eq) of cuprous oxide and 500mL tetrahyd...
Embodiment 2
[0032]
[0033] Put 97.1g (1mol, 1eq) of 1,3-dimethylpyrazole and 700mL ethanol into the reaction flask, and slowly add bromine 158.4g (0.99mol, 0.99eq) dropwise under stirring at 0-5°C. ℃ drop reaction for 1 hour, sample GC material 1 HNMR (400MHz, CDCl 3 ):7.41(s,1H),5.90(s,2H),3.82(s,3H).
[0034]
[0035]Under the protection of nitrogen, put 100g (568.1mmol) of 4-bromo-1-methyl-1H-pyrazol-3-amine and 6mol / L hydrochloric acid aqueous solution into the reaction flask, adjust the pH1 HNMR (400MHz, CDCl 3 ):7.93(s,1H),6.05(s,1H),3.95(s,3H).
[0036]
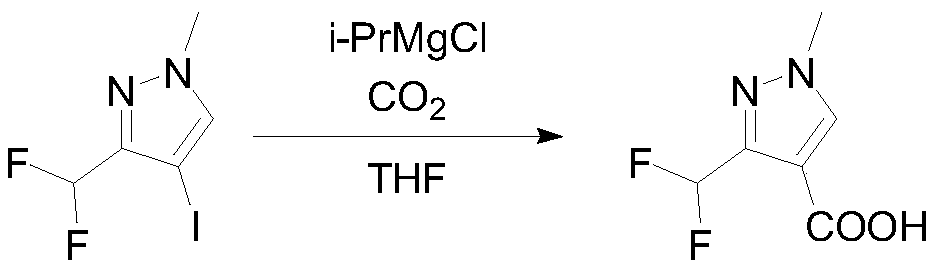
[0037] Under nitrogen protection, 95g (0.368mol, 1eq) of 4-iodo-3-difluoromethyl-1-methylpyrazole and 500mL of 2-methyltetrahydrofuran were put into the reaction flask, stirred evenly, and cooled to -5°C. Slowly add 311 mL of i-PrMgCl-LiCl (1.3M, 1.1 eq) in 2-methyltetrahydrofuran solution dropwise, and keep stirring for 2 hours after dropping to obtain a Grignard exchange solution. Sample was quenched and GC detected...
Embodiment 3
[0039]
[0040] Put 97.1g (1mol, 1eq) of 1,3-dimethylpyrazole and 700mL ethanol into the reaction flask, and slowly add bromine 158.4g (0.99mol, 0.99eq) dropwise under stirring at 0-5°C. ℃ drop reaction for 1 hour, sample GC material 1 HNMR (400MHz, CDCl 3 ):7.41(s,1H),5.90(s,2H),3.82(s,3H).
[0041]
[0042] Under the protection of nitrogen, put 100g (568.1mmol) of 4-bromo-1-methyl-1H-pyrazol-3-amine and 6mol / L hydrochloric acid aqueous solution into the reaction flask, adjust the pH1 HNMR (400MHz, CDCl 3 ):7.93(s,1H),6.05(s,1H),3.95(s,3H).
[0043]
[0044] Under the protection of nitrogen, 95g (0.368mol, 1eq) of 4-iodo-3-difluoromethyl-1-methylpyrazole and 500mL tetrahydrofuran were put into the reaction flask, stirred evenly, and cooled to -5°C. Slowly add 337 mL of tetrahydrofuran solution of s-BuMgCl-LiCl (1.2M, 1.1 eq) dropwise, and keep stirring for 2 hours after dropping to obtain a Grignard exchange solution. Sample was quenched and GC detected 1 HNMR (40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com