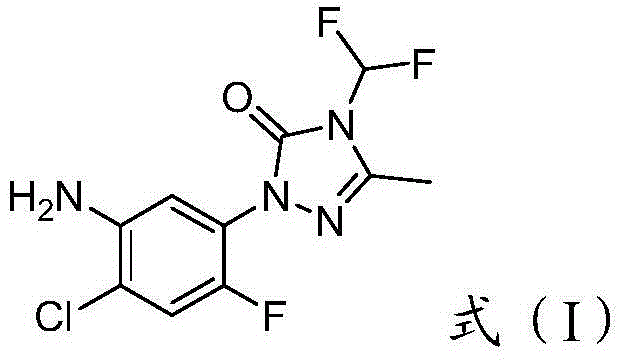
Method for purifying 2-(2-flourine-4-chlorine-5-aminophenyl)-4-difluromethylation-5-methyl-1,2,4-triazole-3-ketone
A technology of aminophenyl and difluoromethyl, which is applied in the field of purification of 2--4--5-methyl-1,2,4-triazol-3-one, can solve the problems of yield reduction, color and The purity is difficult to meet the requirements, the amount of solvent is large, etc., to achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 2-(2-Fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- Add 100mL of 1mol / L hydrochloric acid dropwise to 15g of crude ketone under heating and stirring, the product dissolves, add 20mL of ethyl acetate, stir, let stand, separate the lower aqueous phase, add 1.0g of 350 mesh activated carbon, heat, stir and reflux at 100°C for 20min , remove activated carbon by filtration, add 1mol / L NaOH solution dropwise to the filtrate, until no more precipitation occurs, separate to obtain light yellow solid 2-(2-fluoro-4-chloro-5-aminophenyl)-4-difluoro Methyl-5-methyl-1,2,4-triazol-3-one 8.6 g, melting point 128-129°C.
[0035] 2-(2-fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- Ketone was detected, and the results showed that its purity was 97.6%, and the yield was 82.0%.
Embodiment 2
[0037] 2-(2-Fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- To 15g of crude ketone, add 120mL of 1mol / L hydrochloric acid dropwise under heating and stirring, the product dissolves, add 25mL of ethyl acetate, stir, let stand, separate the lower aqueous phase, add 1.5g of 350 mesh activated carbon, heat, stir and reflux at 100°C for 35min , remove activated carbon by filtration, add 1 mol / L potassium carbonate solution dropwise to the filtrate until no precipitation occurs, and separate to obtain light yellow solid 2-(2-fluoro-4-chloro-5-aminophenyl)-4-di Fluoromethyl-5-methyl-1,2,4-triazol-3-one 9.6 g, melting point 128-129°C.
[0038] 2-(2-fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- Ketone was detected, and the results showed that its purity was 98.2%, and the yield was 85.1%.
Embodiment 3
[0040] 2-(2-Fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- To 20g of crude ketone, add 125mL of 1mol / L sulfuric acid dropwise to it under heating and stirring, the product dissolves, add 40mL of diethyl ether, stir, let stand, separate the lower aqueous phase, add 1.8g of 250-mesh activated carbon, heat and stir at 80°C for 30min, filter to remove Activated carbon, add 1mol / L NaOH solution dropwise to the filtrate until no more precipitation occurs, and separate to obtain a light yellow solid 2-(2-fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl- 15.0 g of 5-methyl-1,2,4-triazol-3-one, melting point 128-129°C.
[0041] 2-(2-fluoro-4-chloro-5-aminophenyl)-4-difluoromethyl-5-methyl-1,2,4-triazole-3- Ketone was detected, and the results showed that its purity was 97.8%, and the yield was 88.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com