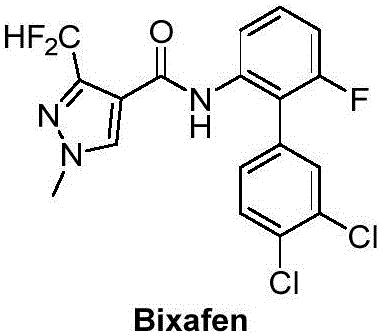
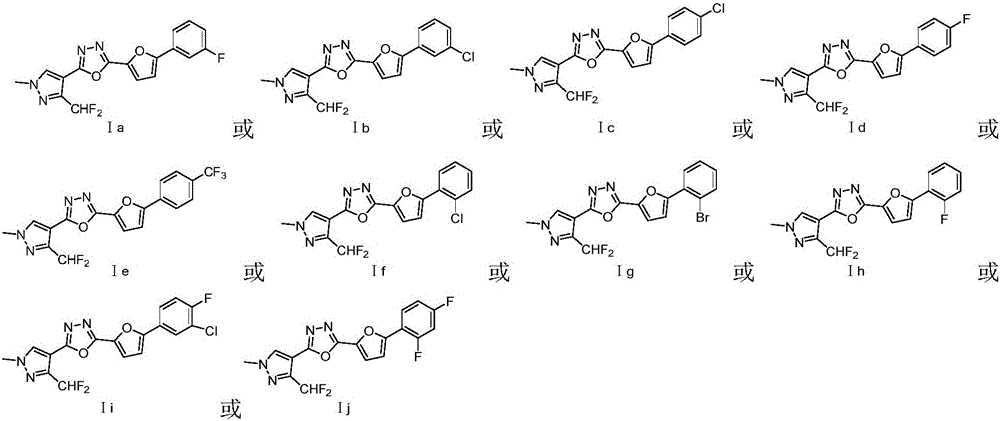
Preparation and application of difluoromethyl pyrazole compound comprising 1,3,4-oxadiazole structure
A technology of difluoromethylpyrazoles and compounds, applied in the field of pesticides, to achieve excellent control effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
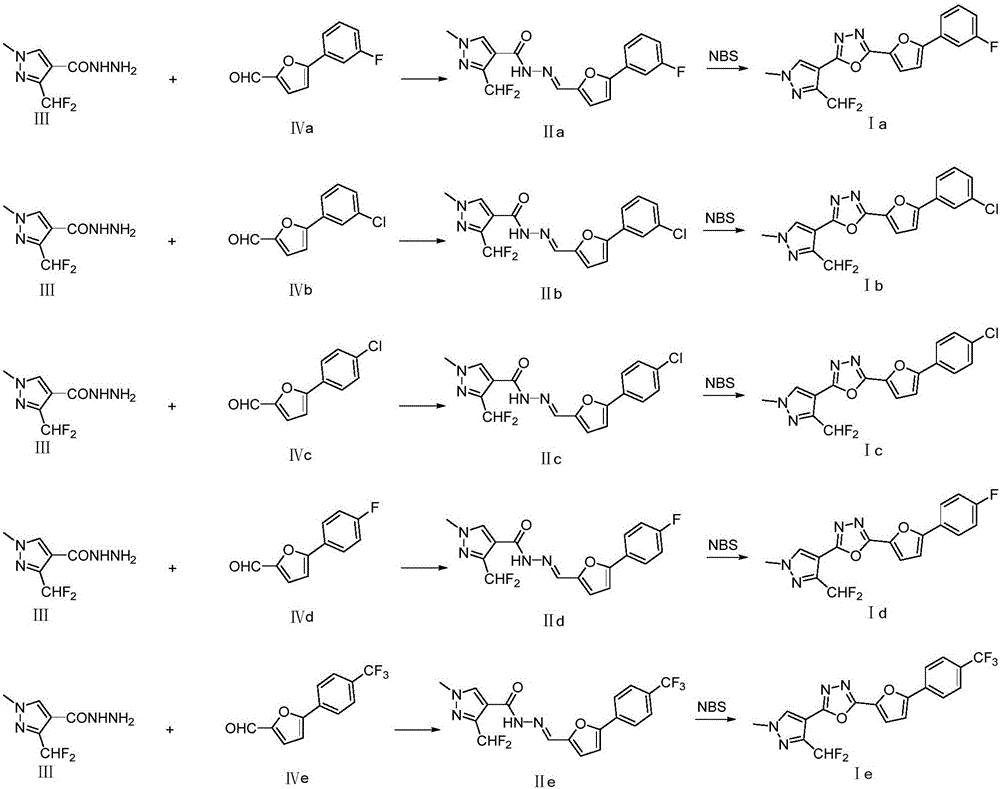
[0023] Embodiment 1: the synthesis of product Ia
[0024]
[0025] Add intermediate III (10mmol) and IVa (10mmol) into 30mL of toluene, raise the temperature to reflux, and use a water separator to continuously take out the water generated by the reaction until no water is produced. After cooling down, concentrate to dryness under reduced pressure. Then the concentrate was dissolved in 30mL chloroform, cooled to 0°C in an ice-water bath, 20mmol N-bromosuccinimide (NBS) was slowly added to the above solution in batches, the reaction was continued at 0°C for 1 hour, and then triethyl Amine 2mL, continue stirring reaction at room temperature for 2 hours, add appropriate amount of water to the reaction mixture, separate the chloroform layer, extract the water phase with chloroform once, combine the chloroform layers, dry over anhydrous sodium sulfate, column chromatography (petroleum ether / acetic acid Ethyl ester=10 / 1) isolated white solid product Ia in 65% yield. 1 HNMR (400M...
Embodiment 2
[0026] Embodiment 2: the synthesis of product Ib
[0027]
[0028] Add intermediate III (10mmol) and IVb (11mmol) to 35mL toluene, raise the temperature to reflux, use a water separator to continuously take out the water generated by the reaction until no water is produced, and after cooling down, concentrate to dryness under reduced pressure. Then the concentrate was dissolved in 25mL chloroform, cooled to 0°C in an ice-water bath, and 25mmol N-bromosuccinimide (NBS) was slowly added to the above solution in batches, and the reaction was continued at 0°C for 1.5 hours, and then triethyl Amine 2mL, continue stirring reaction at room temperature for 2 hours, add appropriate amount of water to the reaction mixture, separate the chloroform layer, extract the water phase with chloroform once, combine the chloroform layers, dry over anhydrous sodium sulfate, column chromatography (petroleum ether / acetic acid Ethyl ester=10 / 1) isolated white solid product Ib with a yield of 55%. ...
Embodiment 3
[0029] Embodiment 3: the synthesis of product Ic
[0030]
[0031] Add intermediate III (10mmol) and IVc (11mmol) into 20mL of toluene, raise the temperature to reflux, and use a water separator to continuously take out the water generated by the reaction until no water is produced. After cooling down, concentrate to dryness under reduced pressure. Then the concentrate was dissolved in 30mL chloroform, cooled to 0°C in an ice-water bath, 22mmol N-bromosuccinimide (NBS) was slowly added to the above solution in batches, the reaction was continued at 0°C for 1 hour, and then triethyl Amine 2.5mL, continue to stir and react at room temperature for 3 hours, add an appropriate amount of water to the reaction mixture, separate the chloroform layer, extract the aqueous phase with chloroform once, combine the chloroform layers, dry over anhydrous sodium sulfate, and perform column chromatography (petroleum ether / Ethyl acetate = 10 / 1) was isolated to obtain the white solid product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com