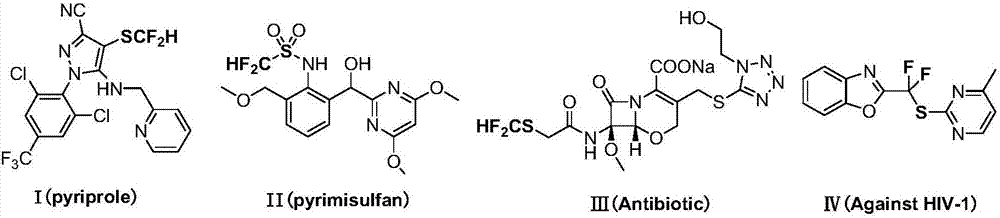
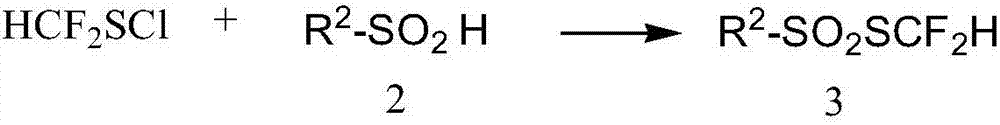Preparation method for difluoromethyl-substituted sulfoaryl sulfonate
A technology of difluoromethyl and sulfonate, which is applied in the direction of organic chemistry, and can solve problems such as the preparation method of thioaryl sulfonate lacking difluoromethyl substitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 difluoromethyl substituted benzylthiol
[0055]
[0056] The preparation of difluoromethyl-substituted benzylthiol can be carried out in any of the following ways.
[0057] Method 1 Add n-hexane (1500mL) into a three-necked flask, place it in a -78°C cold bath for stirring, and slowly introduce chlorodifluoromethane (ClCF 2 H, F-22, 750mmol), NaOH (50g, 1250mmol), tris (3,6-dioxaheptyl) amine (TDA-1, 8.1g, 25mmol) and benzyl mercaptan ( 62.1g, 500mmol), and then slowly raised to 60°C and reacted under dry ice-acetone condensation for 2-4h. The reaction solution was filtered, subjected to desolvation under reduced pressure, and separated by flash silica gel column chromatography to obtain 53 g of light pink oily liquid with a yield of 61%. The hydrogen spectrum showed a purity greater than 98%.
[0058] Method 2 Add KOH (168g, 3000mmol), acetonitrile / water (1400mL, 1:1) and benzyl mercaptan (18.6g, 150mmol) into a three-neck flask, st...
Embodiment 2
[0060] Embodiment 2 Preparation of thiodifluoromethylphenylsulfonate
[0061]
[0062] The preparation of thiodifluoromethylphenyl sulfonate can be prepared by any of the following methods.
[0063] Method 1: Add 98mLCl to the three-necked flask 2 / CHCl 3 solution (1.232mol / L, 120mmol), the flask was placed in a -10°C cold bath and stirred, and the difluoromethyl-substituted benzylthiol (20.9g, 120mmol) prepared in Example 1 was added, and the reaction was carried out for 1h (using 19 F NMR for monitoring). Under a cooling bath at -10°C, sodium phenylsulfinate (19.7 g, 120 mmol) was added rapidly, and then raised to room temperature for reaction. The reaction solution was filtered, subjected to desolvation under reduced pressure, and separated by flash silica gel column chromatography to obtain 19.4 g of a yellow oily liquid with a yield of 72%. The hydrogen spectrum showed a purity greater than 98%.
[0064] Method 2: Add 98mLCl to the three-necked flask 2 / CHCl 3 so...
Embodiment 3
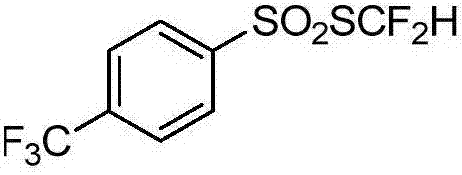
[0071] Example 3 4-Methylthiodifluoromethylarylsulfonate
[0072]
[0073] Add 16.3mLCl to the 50mL sealed tube 2 / CHCl 3 solution (1.232mol / L, 20mmol), the flask was placed in a -10°C cold bath and stirred, and difluoromethyl-substituted benzylthiol 2 (3.48g, 20mmol) was added, and the reaction was carried out for 1h (using 19 F NMR for monitoring). Under a cooling bath at -10°C, sodium 4-methylphenylsulfinate (20 mmol) was added rapidly, and then raised to room temperature for 10 h. The reaction solution was filtered, subjected to desolvation under reduced pressure, and separated by flash silica gel column chromatography to obtain 3.42 g of the corresponding product with a yield of 72%. The hydrogen spectrum showed that the purity was greater than 98%.
[0074] 4-Methylthiodifluoromethylarylsulfonate: 1 H NMR (400MHz, CDCl 3 ,293K,TMS)δ7.47(d,J=8.0Hz,2H),7.16(d,J=8.0Hz,2H),6.80(t,J=56.0Hz,1H),2.35(s,3H)ppm : 19 F NMR (375MHz, CDCl 3 )δ-93.5 (d, J = 56.3Hz, 2F) ppm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com