2-arylsulfonyl-2, 2-difluorodiazoethane compound, preparation method and application thereof
The technology of difluorodiazoethane and arylsulfone group is applied in the field of preparation of diazonium compounds, and can solve the problems of high risk factor and difficult operation, and achieve the effects of simple use, convenient preparation and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example 1: Preparation of 2-phenylsulfone-2,2-difluorodiazoethane compound
[0040]
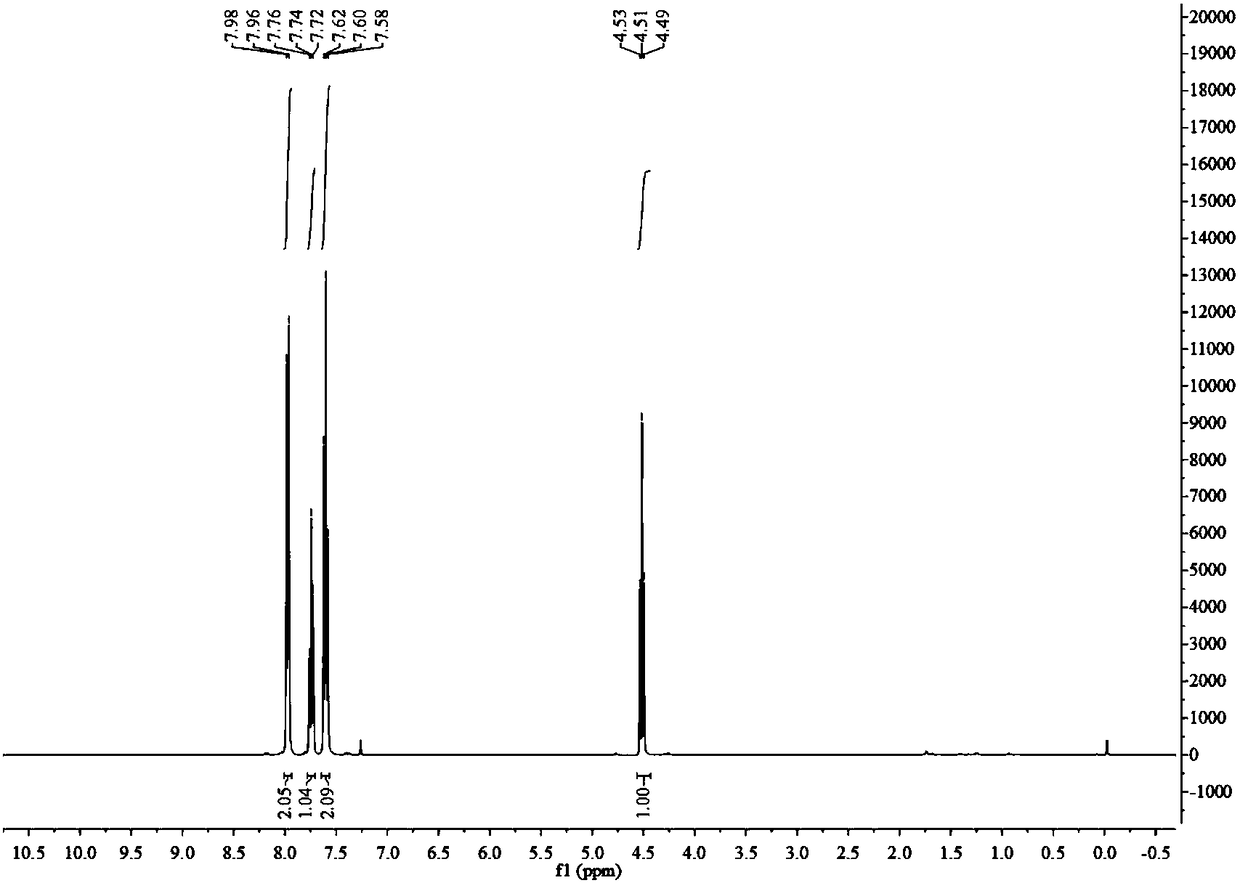
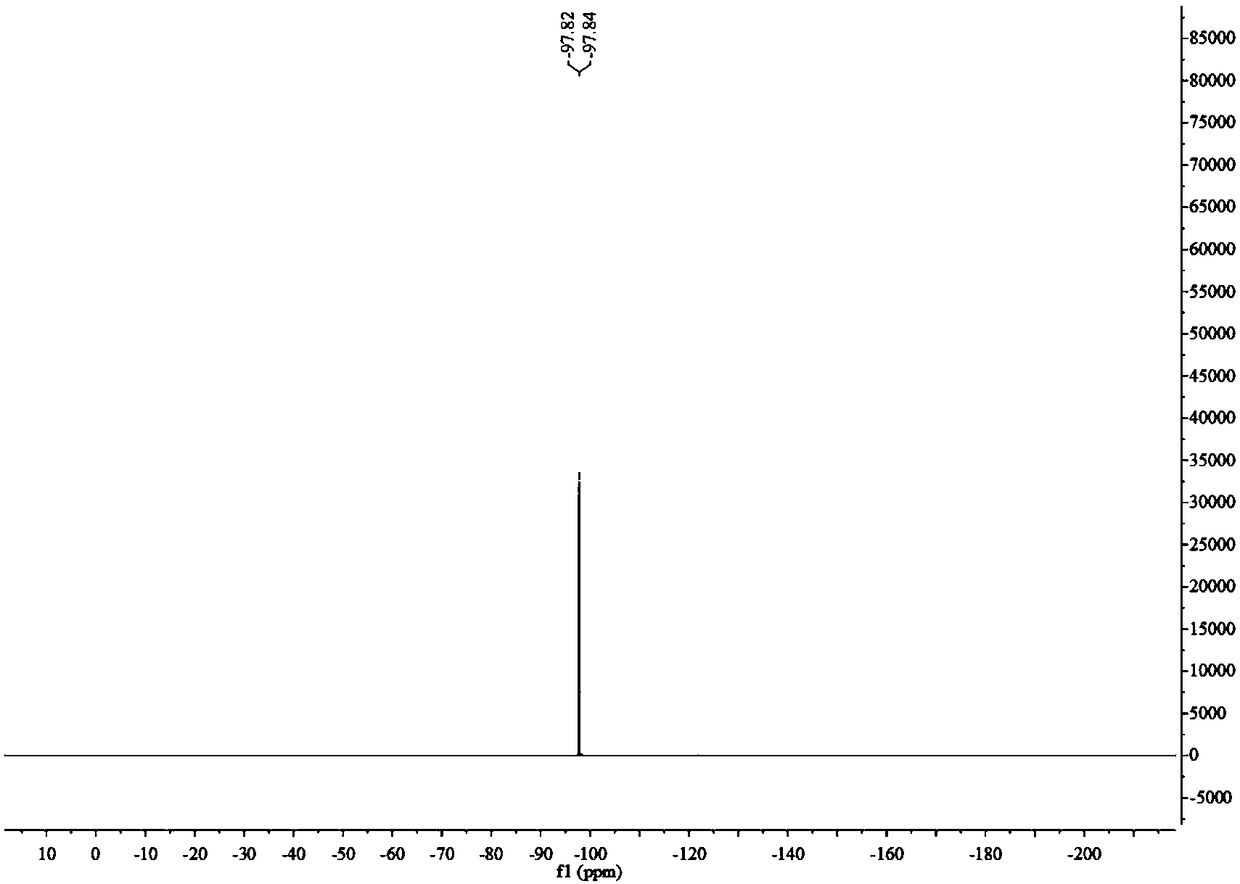
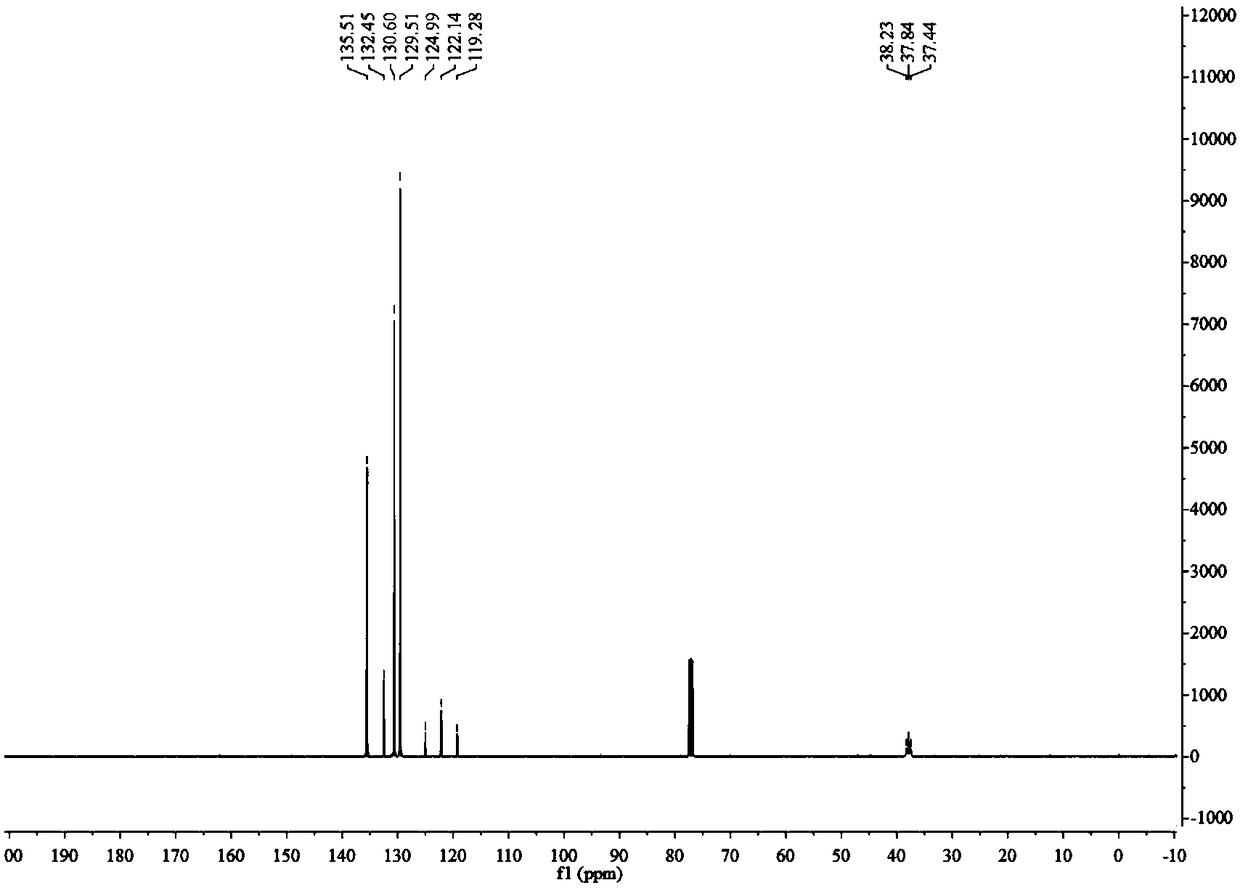
[0041] 1): Dissolve 16.9g of 2,2-difluoro-2-phenylthioethanol in a mixture of 60ml of water and 60ml of acetic acid, add 20mL of 30% hydrogen peroxide, heat and reflux for 3 hours, and the reaction is complete as detected by thin-layer chromatography Afterwards, after washing, extraction and separation, the organic phase was spin-dried to obtain 14 g of white solid 2,2-difluoro-2-phenylsulfone ethanol, with a yield of 85%. 1 H NMR (400MHz, CDCl 3 )δ: 7.99(d, J=7.8Hz, 2H), 7.78(t, J=7.5Hz, 1H), 7.63(t, J=7.8Hz, 2H), 4.29(td, J=12.9, 7.4Hz, 2H), 2.73(t, J=7.3Hz, 1H). 19 F NMR (377MHz, CDCl 3 )δ: -111.19(t,J=12.9Hz). 13 C NMR (101MHz, CDCl 3 )δ: 135.75, 132.34, 130.69, 129.50, 120.74(t, J=289.2Hz), 59.94(t, J=24.9Hz).
[0042] 2): Dissolve 13.5 grams of 2,2-difluoro-2-phenylsulfone ethanol in 100 mL of acetonitrile, add 7 ml of pyridine, add 10.5 mL of trifluoromethanesulfonic anhy...
example 2
[0045] Example 2: Preparation of 2-(4-chlorophenyl)sulfone-2,2-difluorodiazoethane compound
[0046]
[0047] 1): Dissolve 7.8g of 2,2-difluoro-2-(4-chlorophenyl)thioethanol in a mixture of 50ml of water and 50ml of acetic acid, add 14mL of 30% hydrogen peroxide, and heat to reflux for 3 hours. After the completion of the reaction as detected by thin-layer chromatography, washing, extraction, and separation, the organic phase was spin-dried to obtain 8.5 g of white solid 2,2-difluoro-2-(4-chlorophenyl)sulfone ethanol, with a yield of 94%. 1 HNMR (400MHz, CDCl 3 )δ: 7.92(d, J=7.8Hz, 2H), 7.66(d, J=7.8Hz, 2H), 4.4(td, J=11.9, 8.4Hz, 2H), 2.61(t, J=6.3Hz, 1H). 19 F NMR (377MHz, CDCl 3 )δ: -108.19(t,J=10.9Hz). 13 CNMR (101MHz, CDCl 3 )δ: 137.75, 134.34, 131.61, 128.22, 121.74(t, J=279.2Hz), 62.24(t, J=23.9Hz).
[0048] 2): Dissolve 8.5 grams of 2,2-difluoro-2-(4-chlorophenyl)sulfone ethanol in 80ml of acetonitrile, add 4.5ml of pyridine, and add 6.2ml of trifluoromethanes...
example 3
[0050] Example 3: Preparation of 2-(4-fluorophenyl)sulfone-2,2-difluorodiazoethane compound
[0051]
[0052] 1): Dissolve 10.4g of 2,2-difluoro-2-(4-fluorophenyl)thioethanol in a mixture of 60ml of water and 60ml of acetic acid, add 18ml of 30% hydrogen peroxide, and heat to reflux for 3 hours. After the completion of the reaction as detected by thin-layer chromatography, washing, extraction, and separation, the organic phase was spin-dried to obtain 11.5 g of white solid 2,2-difluoro-2-(4-fluorophenyl)sulfone ethanol, with a yield of 96%. 1 HNMR (400MHz, CDCl 3 )δ: 7.89(d, J=9.3Hz, 2H), 7.69(d, J=9.3Hz, 2H), 4.26(td, J=16.9, 7.4Hz, 2H), 2.67(t, J=7.4Hz, 1H). 19 F NMR (377MHz, CDCl 3 )δ: -112.19(t, J=12.9Hz), -101.28(m). 13 C NMR (101MHz, CDCl 3 )δ: 141.24, 137.75, 131.61, 128.22, 122.74 (t, J = 282.3Hz), 58.24 (t, J = 19.9Hz).
[0053] 2): Dissolve 11.5 grams of 2,2-difluoro-2-(4-fluorophenyl)sulfone ethanol in 100ml of acetonitrile, add 6.3ml of pyridine, and add 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com