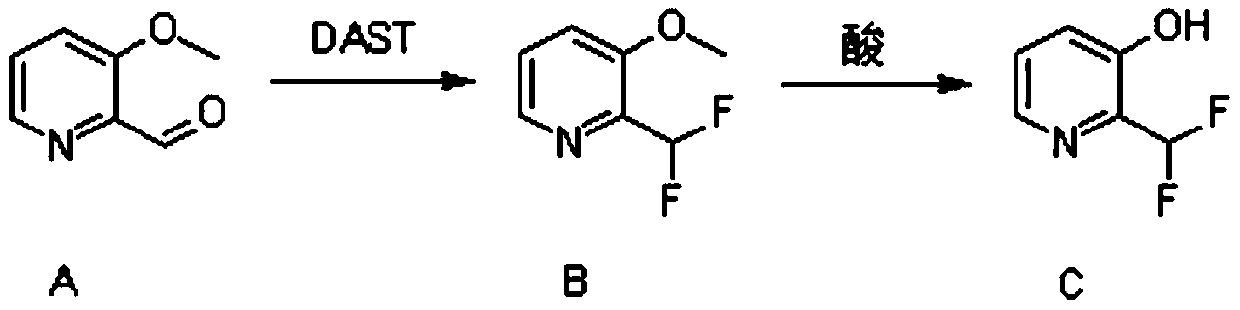
Synthesis method of 2-(difluoromethyl)pyridine-3-ol
A kind of technology of difluoromethyl group and synthetic method is applied in the field of synthesis of 2-pyridine-3-ol, can solve the problems such as the complete route of 2-(difluoromethyl)pyridine-3-ol which is not reported in the literature, etc., and achieves synthesis Simple operation, short process route, easy to achieve effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 3-Methoxypyridine-2-carbaldehyde (24.7g, 180mmol, 1eq.) was dissolved in 480ml of dichloromethane, and cooled to -78°C. Under nitrogen protection, diethylaminosulfur trifluoride (87g, 540mmol, 3eq.) was added dropwise. After the dropwise addition, the reaction was carried out at 25° C. for 15 hours.
[0026] After the reaction, the reaction solution was dropped into ice water and adjusted to alkalinity with saturated sodium bicarbonate solution. After extraction with ethyl acetate, the organic phase was concentrated and subjected to column chromatography to obtain 26.1 g of oily 2-(difluoromethyl)-3-methoxypyridine, with a yield of 91%. used directly in the next reaction.
[0027] Take 95ml of acetic acid and cool it. 190ml mass fraction of 37% hydrobromic acid was added dropwise to the acetic acid and mixed. Then the above mixed acid was added dropwise into the reaction flask of 2-(difluoromethyl)-3-methoxypyridine, the temperature was raised to 115° C., and the re...
Embodiment 2
[0031] 3-Methoxypyridine-2-carbaldehyde (20.6g, 150mmol, 1eq.) was dissolved in 400ml of dichloromethane, and cooled to -78°C. Under nitrogen protection, diethylaminosulfur trifluoride (72.5 g, 450 mmol, 3 eq.) was added dropwise. After the dropwise addition, the reaction was carried out at 5° C. for 30 hours.
[0032] After the reaction, the reaction solution was dropped into ice water and adjusted to alkalinity with saturated sodium bicarbonate solution. After extraction with ethyl acetate, the organic phase was concentrated and subjected to column chromatography to obtain 19.6 g of oily 2-(difluoromethyl)-3-methoxypyridine with a yield of 82%. used directly in the next reaction.
[0033] Take 70ml of acetic acid and cool it. 140ml mass fraction of 37% hydrobromic acid was added dropwise to the above-mentioned acetic acid, and mixed. Then, the above mixed acid was added dropwise into the reaction flask of 2-(difluoromethyl)-3-methoxypyridine, the temperature was raised t...
Embodiment 3
[0037] 3-Methoxypyridine-2-carbaldehyde (27.4g, 200mmol, 1eq.) was dissolved in 520ml of dichloromethane and cooled to -78°C. Under nitrogen protection, diethylaminosulfur trifluoride (96.7g, 600mmol, 3eq.) was added dropwise. After the dropwise addition, the reaction was carried out at 40° C. for 3 hours.
[0038] After the reaction, the reaction solution was dropped into ice water and adjusted to alkalinity with saturated sodium bicarbonate solution. After extraction with ethyl acetate, the organic phase was concentrated and subjected to column chromatography to obtain 23.9 g of oily 2-(difluoromethyl)-3-methoxypyridine, with a yield of 75%. used directly in the next reaction.
[0039] Take 85ml of acetic acid and cool it. 170ml mass fraction of 37% hydrobromic acid was added dropwise to the acetic acid and mixed. Then the above mixed acid was added dropwise into the reaction flask of 2-(difluoromethyl)-3-methoxypyridine, the temperature was raised to 80° C., and the rea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com