N-difluoromethyl hydrazone compound and synthesis method thereof
A technology of difluoromethylhydrazone and its synthesis method, which is applied in the preparation of hydrazone, organic chemistry, sulfonamide preparation, etc., can solve the problems of high toxicity, ozone layer depletion, etc., and achieve no transition metal participation, mild conditions, and substrate adaptation broad effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
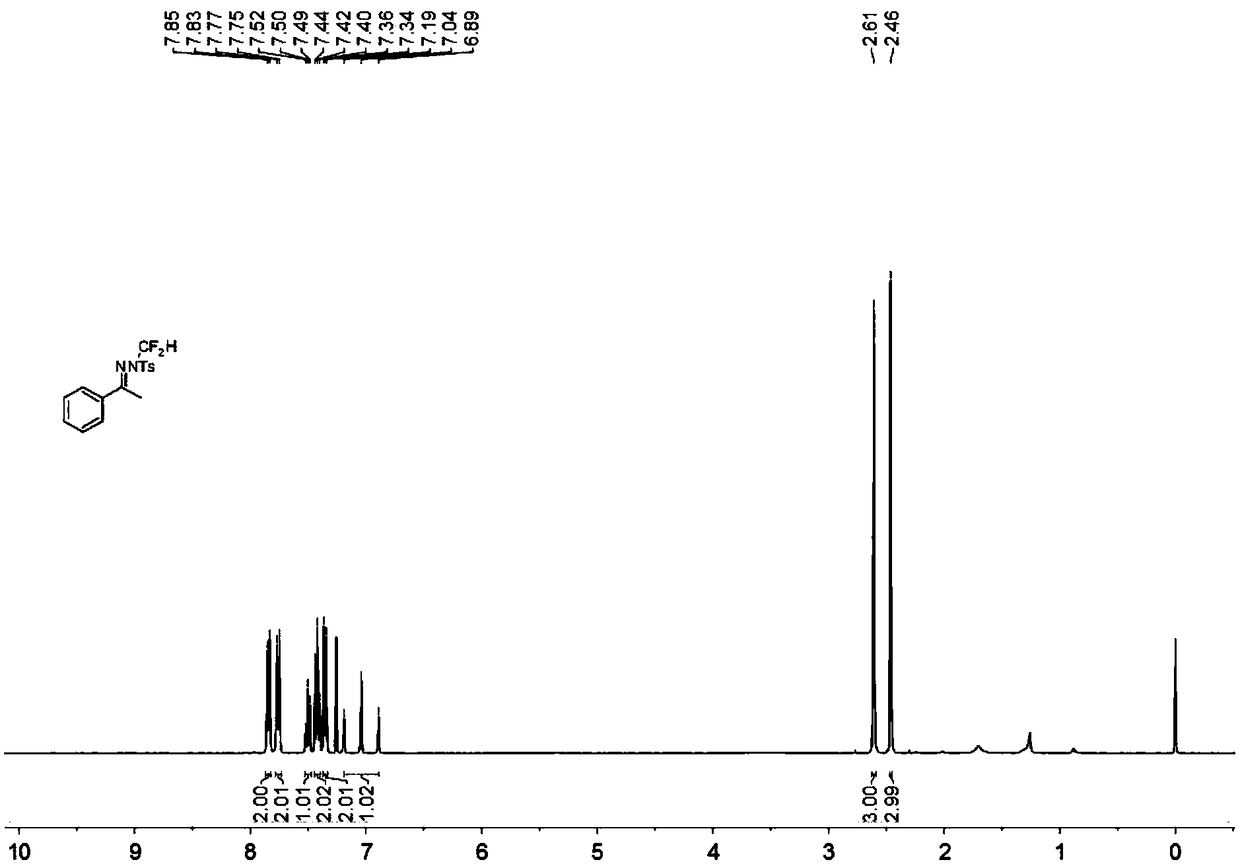
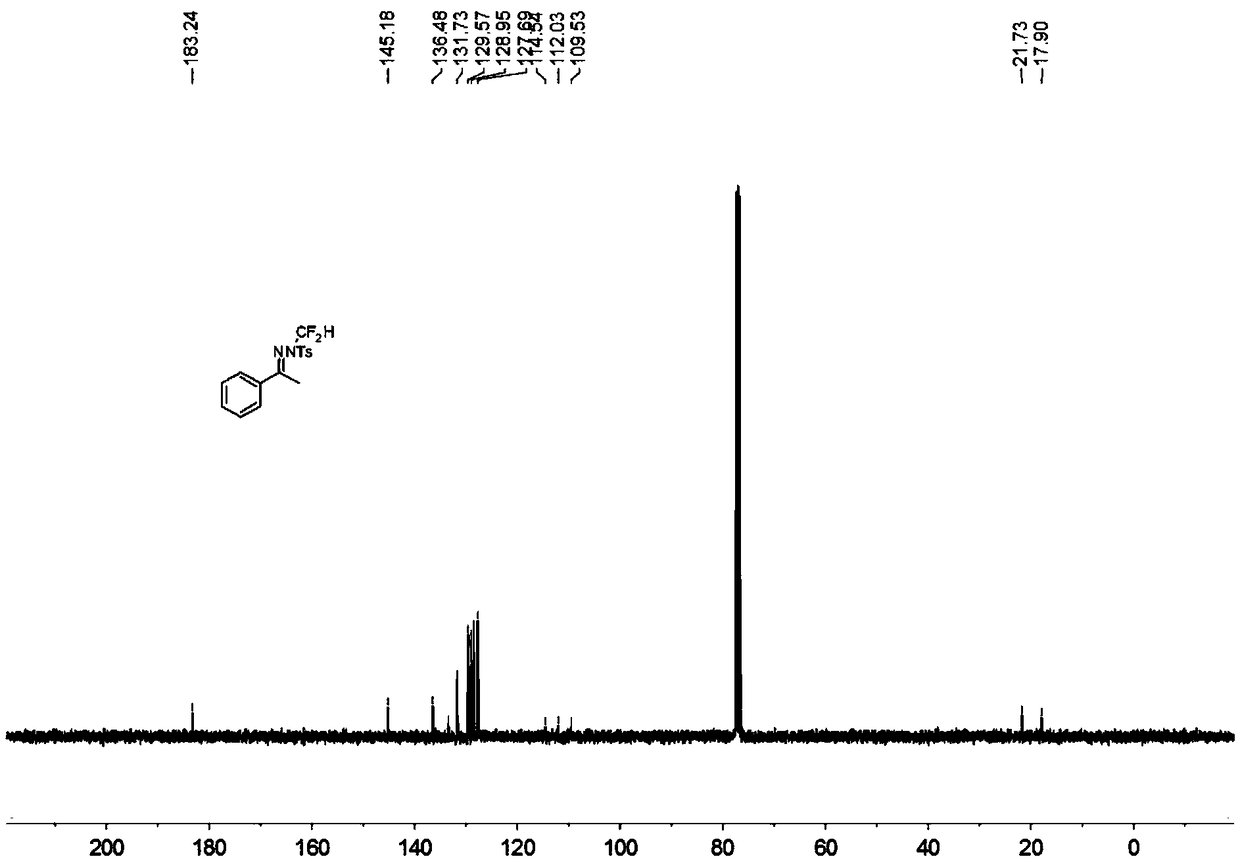
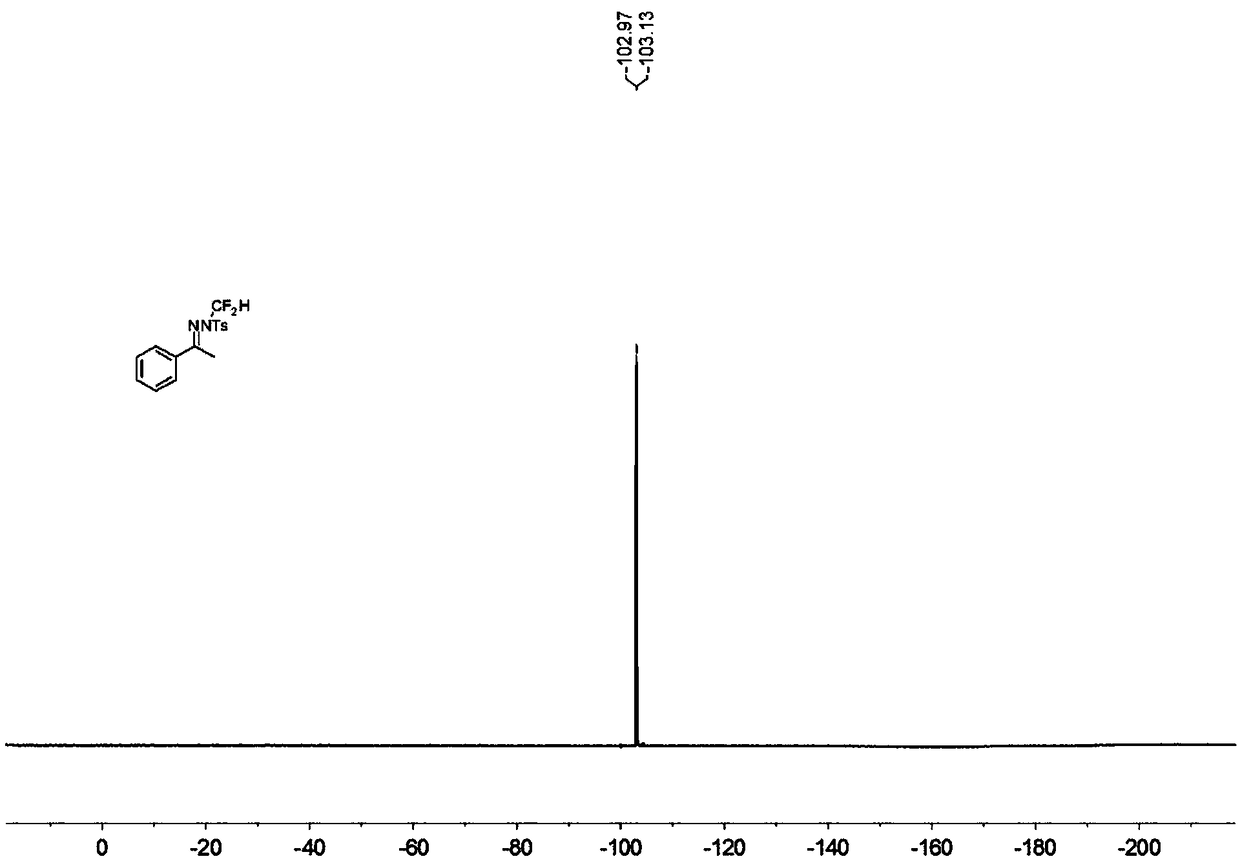
[0037] In the reaction tube, add 0.15 mmol acetophenone p-toluenesulfonylhydrazone, 0.1 mmol bromodifluoromethyltrimethylsilane, 0.225 mmol tert-butoxide lithium, 0.005 mmol benzyltriethylammonium chloride ( Benzyltriethylammoniumchloride, TEBAC) and 1.5 ml of toluene were stirred in an open system at 100° C. at 200 rpm for 12 hours, then stopped heating and stirring, and cooled to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield was 29%.
Embodiment 2
[0039] Add 0.15 mmol of acetophenone p-toluenesulfonylhydrazone, 0.1 mmol of bromodifluoromethyltrimethylsilane, 0.225 mmol of 1,8-diazabicycloundec-7-ene, 0.005 mmoles of benzyltriethylammonium chloride (TEBAC) and 1.5 ml of toluene were stirred and reacted in an open system at 100° C. at a speed of 200 rpm for 12 hours, then heating and stirring were stopped, and cooled to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield was 12%.
Embodiment 3
[0041] Add 0.15 mmol acetophenone p-toluenesulfonylhydrazone, 0.1 mmol bromodifluoromethyltrimethylsilane, 0.225 mmol cesium carbonate, 0.005 mmol tetrabutylammonium fluoride (TBAF) and 1.5 mmol to the reaction tube Milliliter of toluene was stirred in an open system at 100° C. at 200 rpm for 12 hours, then the heating and stirring were stopped, and cooled to room temperature. Add 4 mL of water, extract 3 times with ethyl acetate, combine the organic phases and use 0.5 g of anhydrous magnesium sulfate to dry, filter, evaporate the solvent under reduced pressure, and then separate and purify by column chromatography to obtain the target product The column chromatography eluent used was petroleum ether:ethyl acetate mixed solvent with a volume ratio of 100:1, and the yield was 38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com