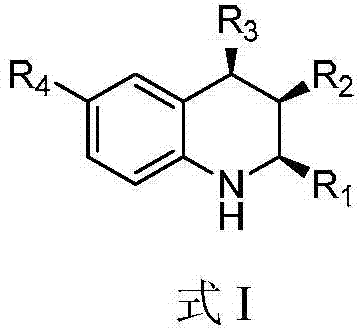
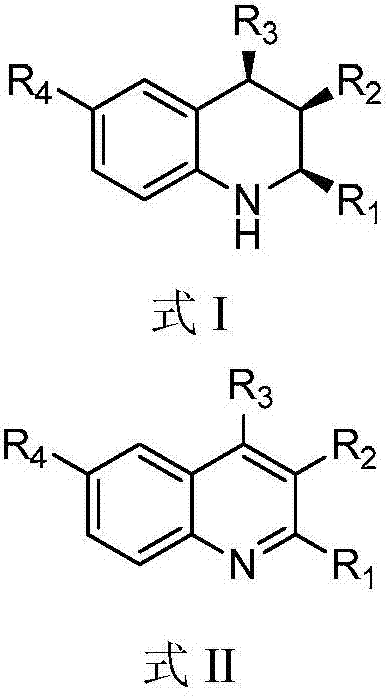
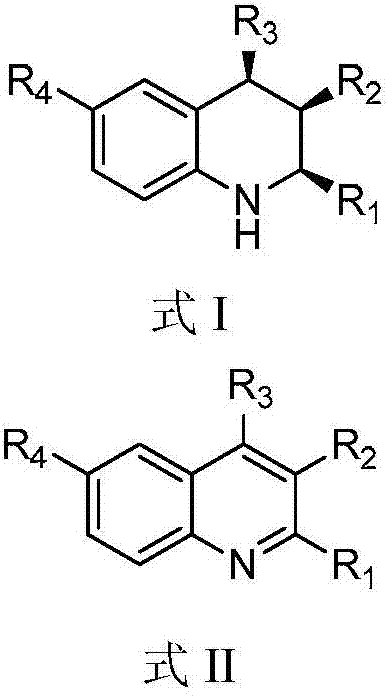
A kind of 1,2,3,4-tetrahydroquinoline derivative and preparation method thereof
A hydrogen and compound technology, which is applied in the field of 1,2,3,4-tetrahydroquinoline derivatives and their preparation, can solve problems such as difficulty in controlling stereoselectivity, and achieve a wide range of substrate use and large industrialization potential , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1, synthesis (+ / -)-(2S,3R,4R)-3-methyl-2,4-diphenyl-1,2,3,4-tetrahydroquinoline and (+)-( 2S,3R,4R)-3-Methyl-2,4-diphenyl-1,2,3,4-tetrahydroquinoline
[0071] (1) Synthesis of (+ / -)-(2S,3R,4R)-3-methyl-2,4-diphenyl-1,2,3,4-tetrahydroquinoline (see structural formula I-a)
[0072]
[0073] Add HB (C 6 f 5 ) 2 (0.02mmol, 0.0069g), styrene (0.02mmol, 0.0021g), toluene (0.6mL), stirred at room temperature for 10 minutes, then added 3-methyl-2,4-diphenylquinoline (formula Ⅱ-a Shown) (0.1180g, 0.4mmol), toluene (0.2mL), then close the reactor, replace the hydrogen three times, and finally fill the hydrogen with a pressure of 20bar, place the reactor in an oil bath at 40°C and stir for 20 hours, Then the hydrogen was evacuated, spin-dried and column chromatography (eluent: petroleum ether: ethyl acetate = 20 / 1, v / v) was a white solid with a yield of 94%.
[0074] The structural verification results are as follows: IR (film): 3370, 1605, 1494, 1477cm -1 ; 1...
Embodiment 2
[0082] Example 2. Synthesis of (+ / -)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methoxyphenyl)-1,2,3,4-tetra Hydroquinoline and (+)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methoxyphenyl)-1,2,3,4-tetrahydroquinoline
[0083] (1) Synthesis of (+ / -)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methoxyphenyl)-1,2,3,4-tetrahydro Quinoline (see structural formula I-b)
[0084] Add HB (C 6 f 5 ) 2 (0.02mmol, 0.0069g), styrene (0.02mmol, 0.0021g), toluene (0.6mL), stirred at room temperature for 10 minutes, then added 3-methyl-4-phenyl-2-(4-methoxybenzene Base)-quinoline (shown in formula II-b) (0.1300g, 0.4mmol), toluene (0.2mL), then close the reactor, pump out the hydrogen for three times, and finally fill in the hydrogen with a pressure of 20bar, and place the reactor Stir in an oil bath at 40°C for 20 hours, then evacuate the hydrogen, spin dry and perform column chromatography (eluent: petroleum ether: ethyl acetate = 20 / 1, v / v) as a white solid with a yield of >99% .
[0085]
[0086] The stru...
Embodiment 3
[0094] Example 3. Synthesis of (+ / -)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methylphenyl)-1,2,3,4-tetrahydro Quinoline and (+)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methylphenyl)-1,2,3,4-tetrahydroquinoline
[0095] (1) Synthesis of (+ / -)-(2S,3R,4R)-3-methyl-4-phenyl-2-(4-methylphenyl)-1,2,3,4-tetrahydroquinone Phenyl (see structural formula I-c)
[0096]
[0097] Add HB (C 6 f 5 ) 2 (0.02mmol, 0.0069g), styrene (0.02mmol, 0.0021g), toluene (0.6mL), stirred at room temperature for 10 minutes, then added 3-methyl-4-phenyl-2-(4-methylphenyl )-quinoline (shown in formula II-c) (0.1236g, 0.4mmol), toluene (0.2mL), then close the reactor, pump out the hydrogen for three times, finally charge into the hydrogen with a pressure of 20bar, place the reactor in Stir in an oil bath at 40°C for 20 hours, then evacuate the hydrogen, spin dry and perform column chromatography (eluent: petroleum ether: ethyl acetate = 20 / 1, v / v) as a white solid with a yield of 91%.
[0098] The structure con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com