Synthesis method of N2-beta-sulfanyl triazole derivative
A technology of sulfanyl triazoles and synthetic methods, applied in organic chemistry methods, organic chemistry, etc., can solve the problems of reduced aromaticity of quinone structure, limited application range of derivatives, difficulty in realization, etc., and achieve universality of reaction Strong, easy to operate, no effect of transition metal participation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
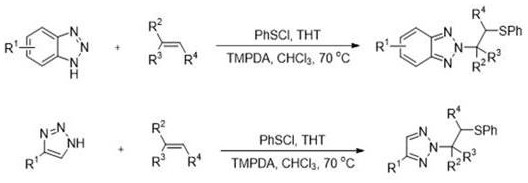
[0018] N 2 When the sulfur source benzene sulphenyl chloride in a solvent Ⅰ, the tetrahydrothiophene absence of a catalyst or catalyst role, or in the presence of a base: -β- synthetic methods sulfanyl triazole derivatives, comprising the steps of absence of a base, a triazole, a sulfur source, an olefin, a reaction at 50-80 ℃ 12-36 h, was concentrated in vacuo and the title compound obtained by column chromatography; triazole is a benzotriazole, a benzotriazole derivative thereof, 1,2,3-triazole or 1,2,3-triazole derivative.
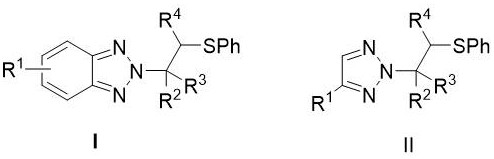
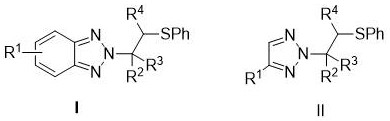
[0019] N 2 The structure of the -β-thioalkyl triazole derivative is as shown in formula I and II:
[0020]
[0021] Rage I and II 1 Hydrogen, methyl, n-butyl, halogen, nitro, carboxy, phenyl, phenyl, methylene, nitro-substituted phenyl group or halogen substituted phenyl; 2 For phenyl, acetyloxy phenyl, chloromethylphenyl, halogenated phenyl, phenyl substituted phenyl, methyl substituted phenyl, tert-butyl substituted phenyl, methoxy substituted phenyl , ...
Embodiment 1
[0037] Chloroform as a solvent, 0.05 mmol tetrahydrothiophene (THT) as catalyst, 0.25 mmol N, N, N ', N'- tetramethyl-propanediamine (TMPDA) is an alkali, 0.5 mmol benzotriazole, 1.0 mmol PhSCl and 1.5 mmol of styrene was reacted at 70 ℃ 36 h, after column chromatography and concentrated to give the title compound can be A, a yield of 86%, N 2 - selectivity 88%.
[0038] 1 H NMR (400 MHz, CDCl 3 ) Δ 7.85 (dd, J = 6.6, 3.1 Hz, 2H), 7.44 (dd, J = 7.7, 1.5 Hz, 2H), 7.39 - 7.15 (m, 11H), 6.04 (dd,J = 9.6, 5.5 Hz, 1H), 4.25 (dd, J = 14.1, 9.6 Hz, 1H), 3.78 (dd, J = 14.1, 5.5 Hz, 1H); 13 C NMR (100MHz, CDCl 3 ) Δ 144.2, 137.6, 134.3, 131.3, 129.0, 128.8, 128.1, 127.2, 127.1,126.3, 118.2, 70.1, 40.0.
Embodiment 2
[0040] The specific synthetic steps of Reference Example 1, the triazole is a benzotriazole, an olefin of 4-acetoxystyrene.
[0041] B Yield 66%, N 2 - selectivity was 84%.
[0042] 1 H NMR (400 MHz, CDCl 3 ) Δ 7.84 (dd, J = 6.6, 3.1 Hz, 2H), 7.47 (d, J = 8.6 Hz, 2H), 7.36 (dd, J = 6.7, 3.0 Hz, 4H), 7.28 - 7.22 (m, 2H), 7.20 (t, J = 7.2 Hz, 1H), 7.04 (d, J = 8.6 Hz, 2H), 6.03 (dd, J = 9.6, 5.4 Hz, 1H), 4.23 (dd, J = 14.2, 9.7 Hz, 1H), 3.75 (dd, J = 14.2, 5.4 Hz, 1H), 2.25 (s, 3H). 13 CNMR (100 MHz, CDCl 3 ) Δ 169.1, 150.9, 144.2, 135.1, 134.1, 131.3, 129.1,128.5, 127.3, 126.5, 122.0, 118.2, 69.5, 40.1, 21.0.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com