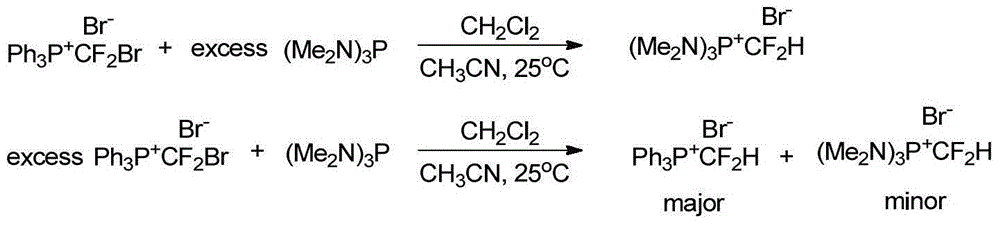
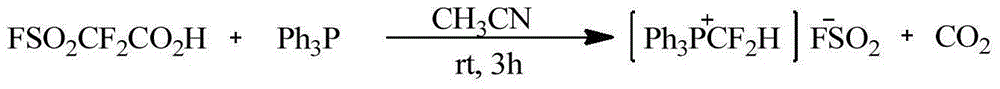Difluromethylphosphonium salt, and preparation method and application thereof
A technology of difluoromethylphosphonium salt and difluoromethylenephosphonium, which is applied in the field of difluoromethylphosphonium salt and its preparation and application, and can solve the problems of expensive, cumbersome preparation methods, harsh reaction conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis
[0078] In a 100mL single-necked bottle, first add difluoromethylphosphonium inner salt ( 7.12g, 0.02mol), then add THF (50mL), under stirring, slowly add 40% aqueous hydrogen bromide (2.9mL, 0.024mol), when the acid is added, all the solids are dissolved, and under stirring Under reflux for 1 hour, the reaction was stopped; the system was cooled to room temperature and then concentrated with a rotary evaporator; then ether (20 mL) was added to the concentrated solution, stirred for a while, the solution was divided into two layers, and the upper layer solution was poured out, and so on. Five times; THF (20 mL) was added and stirred until a white solid precipitated, filtered under reduced pressure, washed three times with THF (5 mL), and finally a white solid was obtained with a yield of 90% and a purity >99.9% (NMR purity).
[0079] white solid, 1 H NMR (400MHz, DMF) δ9.50 (td, J = 46.6, 29.6Hz, 1H), 8.16–8.06 (m, 9H), 7.94 (m, 6H). 19 F NMR (376MHz, D...
Embodiment 2
[0083] Synthesis
[0084] In a 100mL single-necked bottle, first add difluoromethylphosphonium inner salt ( 7.12g, 0.02mol), then add THF (50mL), under stirring, slowly add 60% hexafluorophosphoric acid aqueous solution (2.4mL, 0.024mol), when the acid is added, all the solids dissolve, and under stirring Under reflux for 1 hour, the reaction was stopped; the system was cooled to room temperature and then concentrated with a rotary evaporator; then ether (20 mL) was added to the concentrated solution, stirred for a while, a white solid was precipitated, and the ether solution was poured out, and this was repeated three times Add THF (20mL) and stir, filter under reduced pressure, wash with THF (5mL) three times, and finally obtain a white solid. The resulting solid was dissolved in dichloromethane (10 mL), ion-exchanged with 1 mol / L potassium hexafluorophosphate aqueous solution (10 mL×3), and finally the dichloromethane was removed under reduced pressure to obtain the pro...
Embodiment 3
[0087] Synthesis.
[0088] In a 100mL single-necked bottle, first add difluoromethylphosphonium inner salt ( 7.12g, 0.02mol), then THF (50mL) was added, under stirring, bis(trifluoromethanesulfonyl)imide (6.7g, 0.024mol) was added slowly, when the imine was added, all the solids were dissolved , refluxed for 1 hour under stirring, and stopped the reaction; the system was cooled to room temperature and then concentrated with a rotary evaporator; then, ether (20 mL) was added to the concentrated solution, stirred for a while, and a white solid was precipitated, and the ether solution was poured out , so repeated three times; add THF (20mL) and stir, filter under reduced pressure, wash with THF (5mL) three times, and finally obtain a white solid with a yield of 70% and a purity of >99.9% (NMR purity).
[0089] White solid, M.P.:75.0-75.5℃. 1 H NMR (400MHz, DMF): δ9.50(td, J=46.8, 29.6Hz, 1H), 8.16–8.06(m, 9H), 7.94(m, 6H). 19 F NMR (376MHz, DMF): δ-127.25 (dd, J=77.7, 46.8H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com