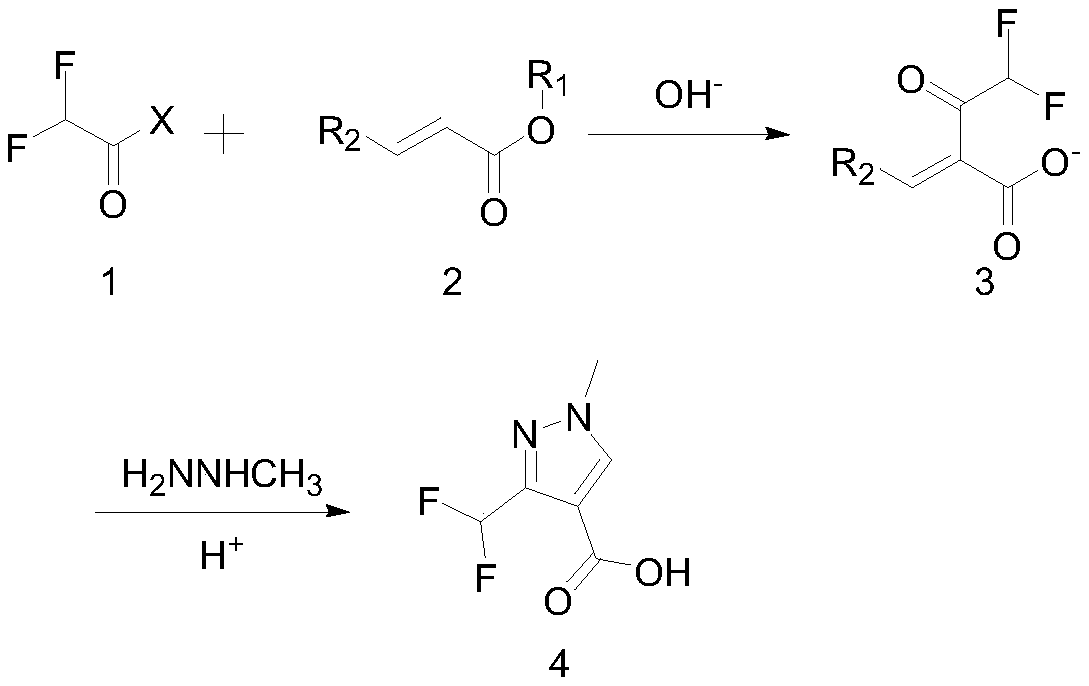
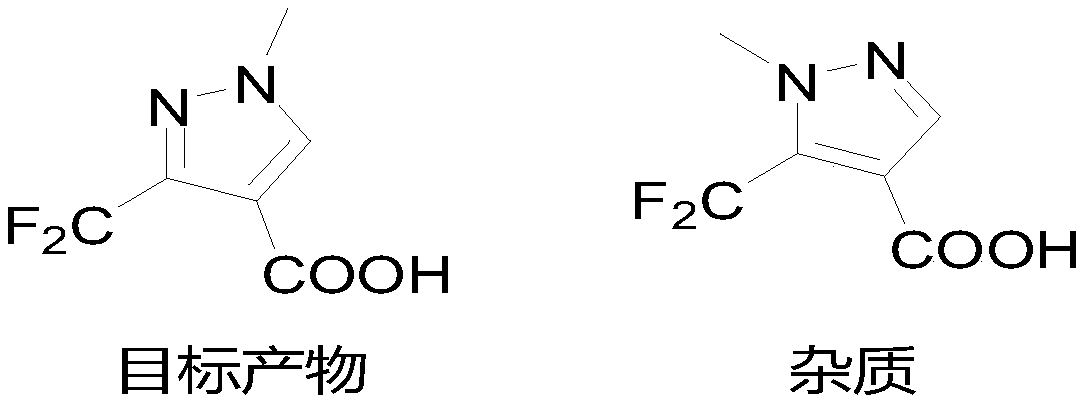
Preparation method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
A technology of difluoromethyl and dimethylamino groups, which is applied in the field of preparation of 3--1-methyl-1H-pyrazole-4-carboxylic acid, and can solve problems such as cumbersome operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033]
[0034] Under nitrogen protection, put 393g (2.5mol, 1eq) of N,N-dimethylaminoisopropyl acrylate, 303.6g (3.0mol, 1.2eq) of triethylamine and 3000mL of 1,4-dioxane into the reaction bottle . Control the reaction temperature at -10-5°C, slowly add 235.3g (2.4mol, 0.96eq) of 2,2-difluoroacetyl fluoride dropwise, react at -5-5°C for 1 hour after the addition, and detect the raw materials by GC <0.5%, quenched by adding 500g of water, standing for stratification, cooling the organic phase to -5-5°C, adding 1000g of 10% NaOH aqueous solution dropwise, reacting at -5-5°C for 1 hour after the dropwise addition, and obtaining The mixed solution of sodium 2-difluoroacetyl-3-(dimethylamino)acrylate was stored under nitrogen at 5-5°C until use.
[0035]
[0036] Under the protection of nitrogen, add 187.4g (1.25mol, 0.5eq) of sodium iodide to the above mixed solution of sodium 2-difluoroacetyl-3-(dimethylamino)acrylate, cool the reaction solution to -30°C, add dropwise 40...
Embodiment 2
[0038]
[0039] Under nitrogen protection, put 428.1g (2.5mol, 1eq) of N,N-dimethylaminoacrylate tert-butyl ester, 355.4g (2.75mol, 1.1eq) of N,N-diisopropylethylamine and di Chloromethane 3500mL. Control the reaction temperature at -10-5°C, slowly add 280.5g (2.45mol, 0.98eq) of 2,2-difluoroacetyl chloride dropwise, and react at -5-5°C for 1 hour after the addition, the raw material is detected by GC < 0.5%, quenched by adding 500g of water, standing to separate layers. Cool the organic phase to -5-5°C, add 700g of 20% KOH aqueous solution dropwise, react at -5-5°C for 1 hour after the dropwise addition, and obtain 2-difluoroacetyl-3-(dimethylamino) after filtration Potassium acrylate mixed solution, and stored under nitrogen at 5-5 ° C until use.
[0040]
[0041]Under the protection of nitrogen, 249g (1.5mol, 0.6eq) of potassium iodide was added to the above mixed solution of potassium 2-difluoroacetyl-3-(dimethylamino)acrylate, the temperature of the reaction solut...
Embodiment 3
[0043]
[0044] Under nitrogen protection, 393g (2.5mol, 1eq) of N,N-methyl diethylaminoacrylate, 303.6g (3mol, 1.2eq) of triethylamine and 3500mL of tetrahydrofuran were put into the reaction flask. Control the reaction temperature at -10-5°C, slowly add 235.3g (2.4mol, 0.96eq) of 2,2-difluoroacetyl fluoride dropwise, react at -5-5°C for 1 hour after the addition, and detect the raw materials by GC <0.5%, quenched by adding 500g of water, and allowed to stand for stratification. Cool the organic phase to -5-5°C, add 1000g of 10% NaOH aqueous solution dropwise, react at -5-5°C for 1 hour after the dropwise addition, and obtain 2-difluoroacetyl-3-(diethylamino) after filtration The sodium acrylate mixed solution was stored under nitrogen at 5-5°C until use.
[0045]
[0046] Under the protection of nitrogen, add 187.4g (1.25mol, 0.5eq) of sodium iodide to the above mixed solution of sodium 2-difluoroacetyl-3-(diethylamino)acrylate, cool the reaction solution to -30°C, ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com