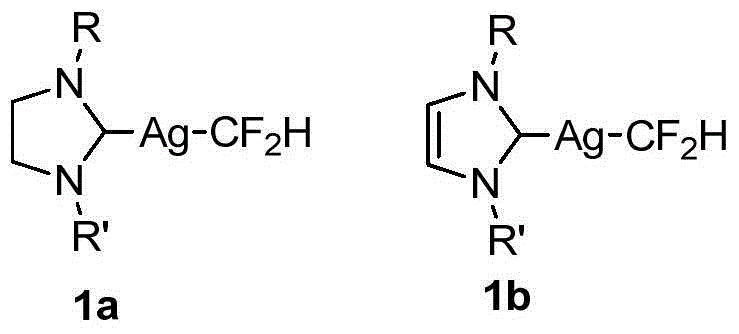
Difluoromethyl silver compound, single crystal, synthetic method and application
A technology of difluoromethyl silver and dimethyl silver, applied in silver organic compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problem of low yield, difficulty in synthesizing difluoromethyl compounds, long routes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
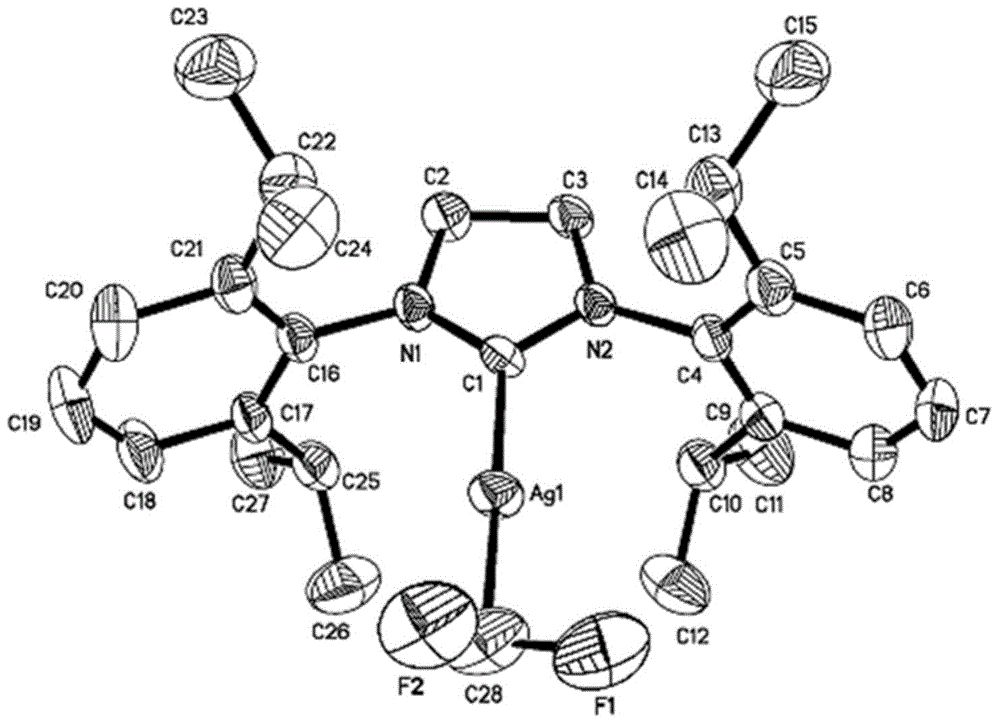
[0109] Example 1 [(SIPr)AgCF 2 Preparation of H]1a-1
[0110]
[0111] [(SIPr)AgCl] (831mg, 1.50mmol) and NaO under argon atmosphere t Bu (285mg, 3.00mmol) was dissolved in THF (30mL), TMSCF was added at room temperature 2 H (375uL, 3.00mmol). Stirred at room temperature in the dark for 1 hour under the protection of Ar, filtered with diatomaceous earth, and the filtrate was dried under reduced pressure. The obtained yellow solid was recrystallized under the condition of dichloromethane / n-pentane to obtain a white solid [(SIPr )AgCF 2 H] 1a-1 (698mg, 82%). Purity identified by hydrogen spectrum greater than 95%.
[0112] 1 H NMR (400MHz,THF-d 8 )δ7.36(t,J=8.0Hz,2H),7.26(d,J=8.0Hz,2H),5.90(td,J=52,14Hz,1H),4.04(s,4H),3.15(hept ,J=6.8Hz,4H),1.34(d,J=7.2Hz,12H),1.32(d,J=7.2Hz,12H);
[0113] 19 F NMR (376MHz,THF-d 8 )δ-113.66(dd,J 109 Ag-F =64.0Hz,J 107 Ag-F =56.4Hz,J H-F =45.1Hz); 13 C NMR (101MHz, CDCl 3 )δ24.11, 25.76, 28.96, 124.55, 129.78, 134.80, 146.76, 1...
Embodiment 2
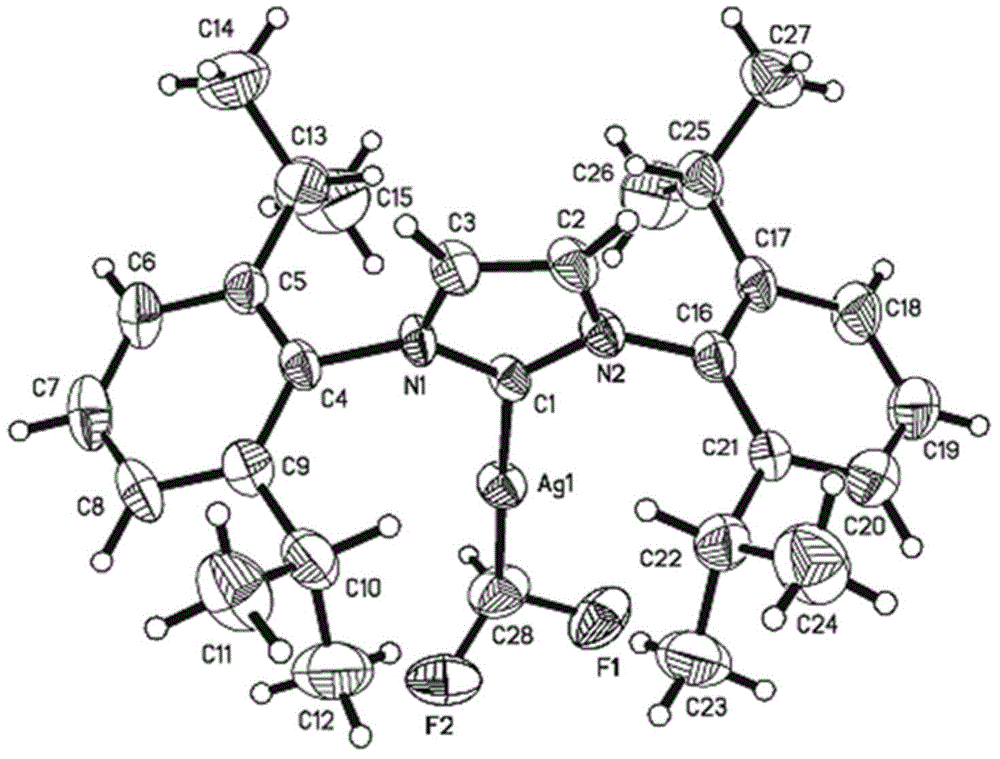
[0115] Example 2 [(IPr)AgCF 2 Preparation of H]1b-1
[0116]
[0117] [(IPr)AgCl] (552mg, 1.00mmol) and KO under argon atmosphere t Bu (225mg, 2.00mmol) was dissolved in THF (90mL) and TMSCF was added 2 H (250 μL 3.00 mmol) in THF (10 mL). Under the protection of argon, stir at room temperature in the dark for 4 hours, then filter with diatomaceous earth, and vacuum the filtrate to dryness. The obtained yellow solid is recrystallized under the condition of dichloromethane / n-pentane to a white solid [(IPr)AgCF 2 H] 1b-1 (189mg, 35%). The purity is greater than 95% identified by hydrogen spectrum.
[0118] 1 H NMR (400MHz, CDCl 3 )δ7.48(t,J=7.6Hz,2H),7.28(d,J=7.6Hz,2H),7.14(s,2H),6.25(td,J=64.0,16.0Hz),2.53(hept, J=7.2Hz,4H),1.26(d,J=6.8Hz,12H),1.20(d,J=6.8Hz,12H);
[0119] 19 F NMR (376MHz, CDCl 3 )-112.76(dd,J 109 Ag-F =63.9Hz,J 107 Ag-F =56.2,J H-F =41.4Hz). 13 C NMR (126MHz, CDCl 3 ) 23.94, 24.54, 28.68, 123.23, 124.10, 125.03, 130.39, 134.67, 145.61, 155....
Embodiment 3
[0125] Example 3 Difluoromethyl metal silver compound 1a-1 (SIPrAgCF 2 Study on the reactivity of H[SIPr=1,3-bis(2,6-diisopropylphenyl)imidazole])
[0126] First, we used the difluoromethylation reaction of difluoromethyl metal silver compound and diaryl iodide, and through the screening of conditions (see Table 2 for details), we determined that the difluoromethyl metal silver compound with two equivalents , two equivalents of cuprous iodide, and acetonitrile as a solvent, the difluoromethylation reaction of diaryl iodides was realized at room temperature.
[0127]
[0128] Table 2 Screening of reaction conditions for diaryl iodides
[0129] Numbering
[0130] Reaction conditions: a) the diaryl iodide of 0.05mmol, the difluoromethyl silver of 0.05mmol, the cuprous iodide of 0.05mmol are dissolved in the acetonitrile of 0.5ml; b) take trifluoromethoxybenzene as internal standard Fluorine spectrum yield; c) 5 equivalents of cuprous iodide added; d) 2 equivalents...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com