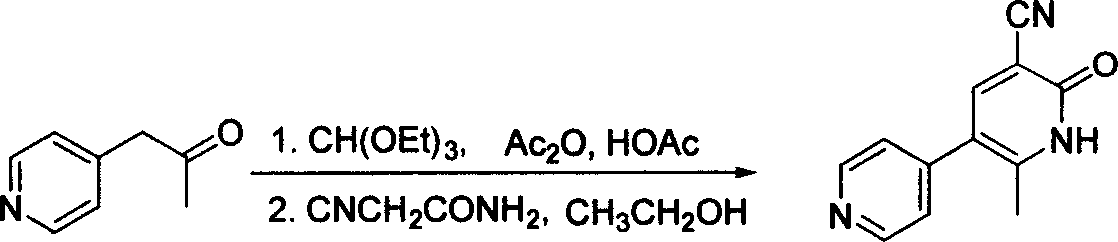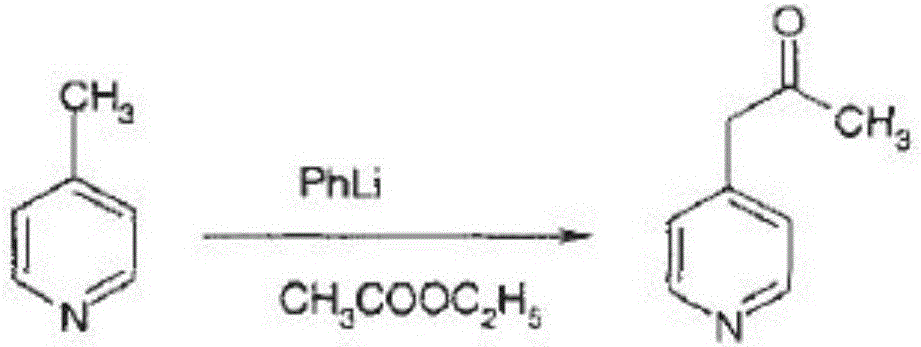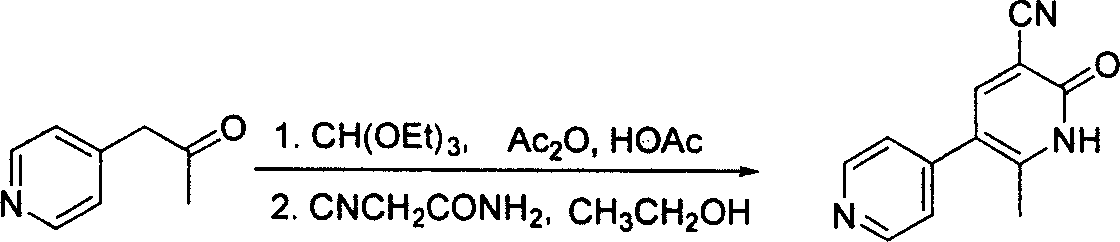Patents
Literature
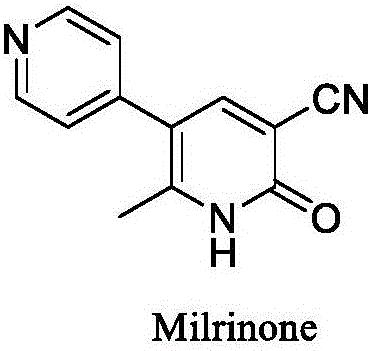
60 results about "Milrinone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
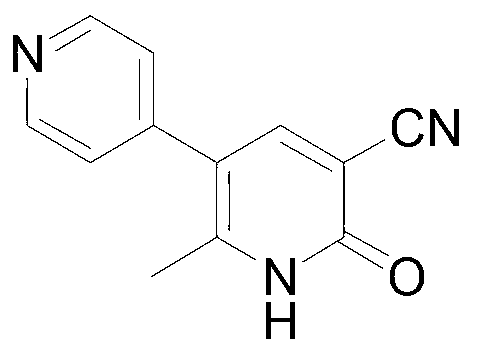
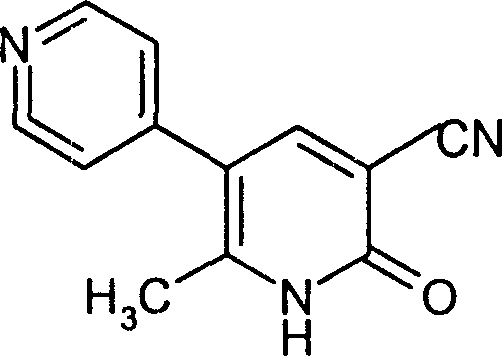
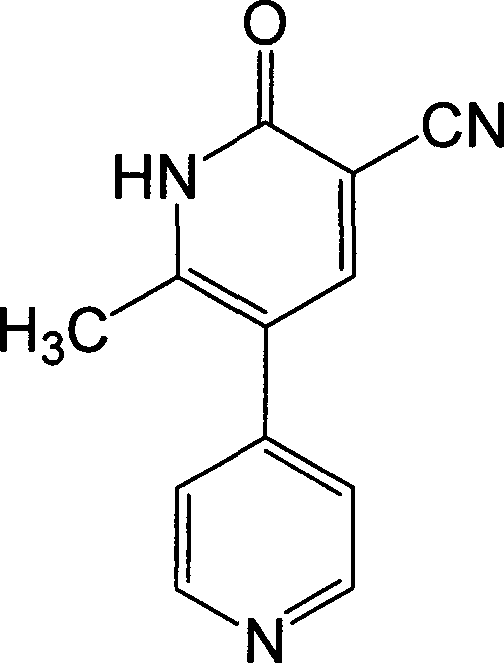
This medication is used for the short-term treatment of heart failure.
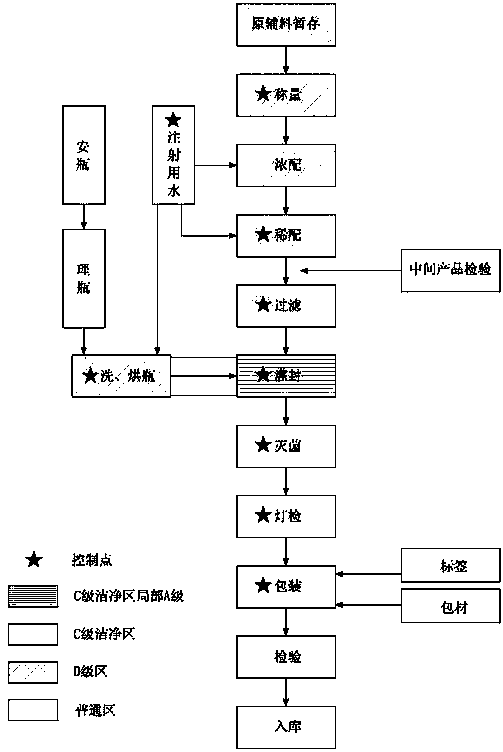
Milrinone sodium chloride injection and production thereof
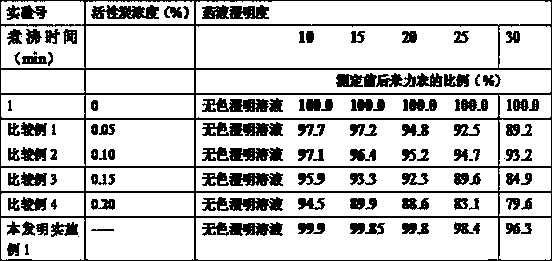
ActiveCN1679566AImprove toleranceSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneSodium Chloride Injection
A milrinone-sodium chloride injection for treating congestive heart failure is proportionally prepared from milrinone, sodium chloride and lactic acid.
Owner:LUNAN BETTER PHARMA
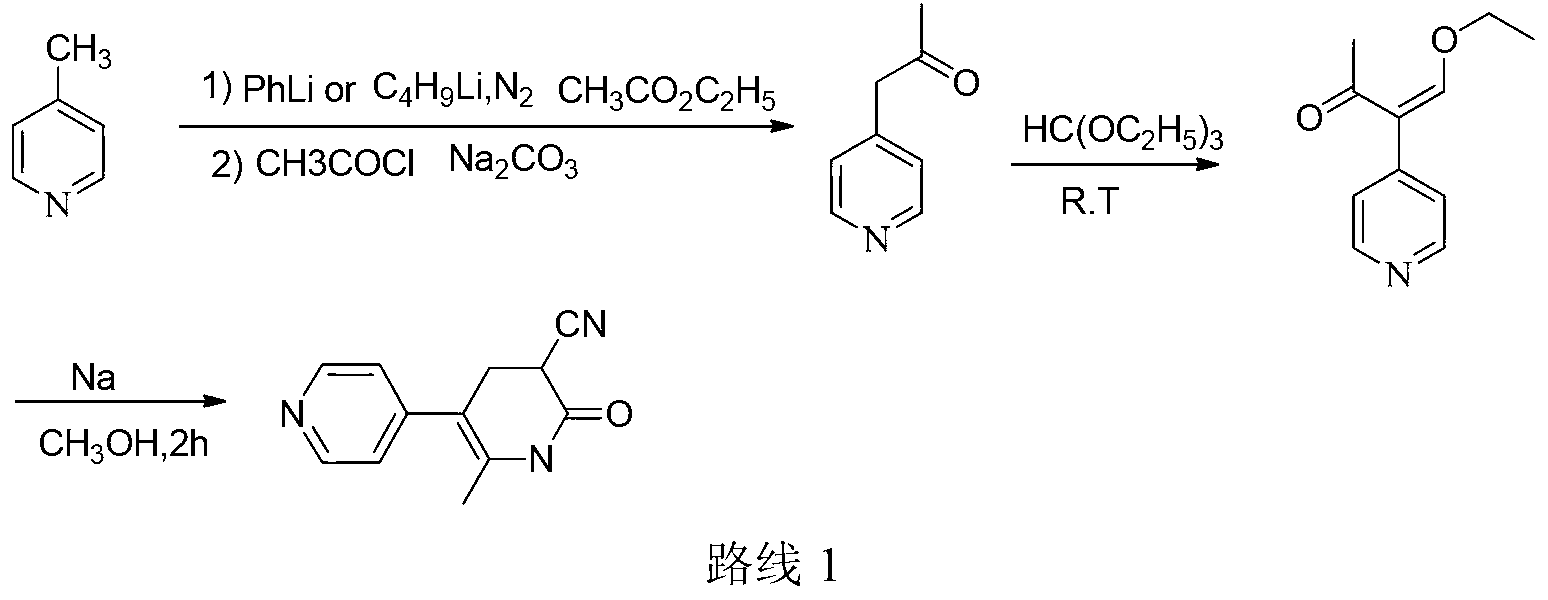
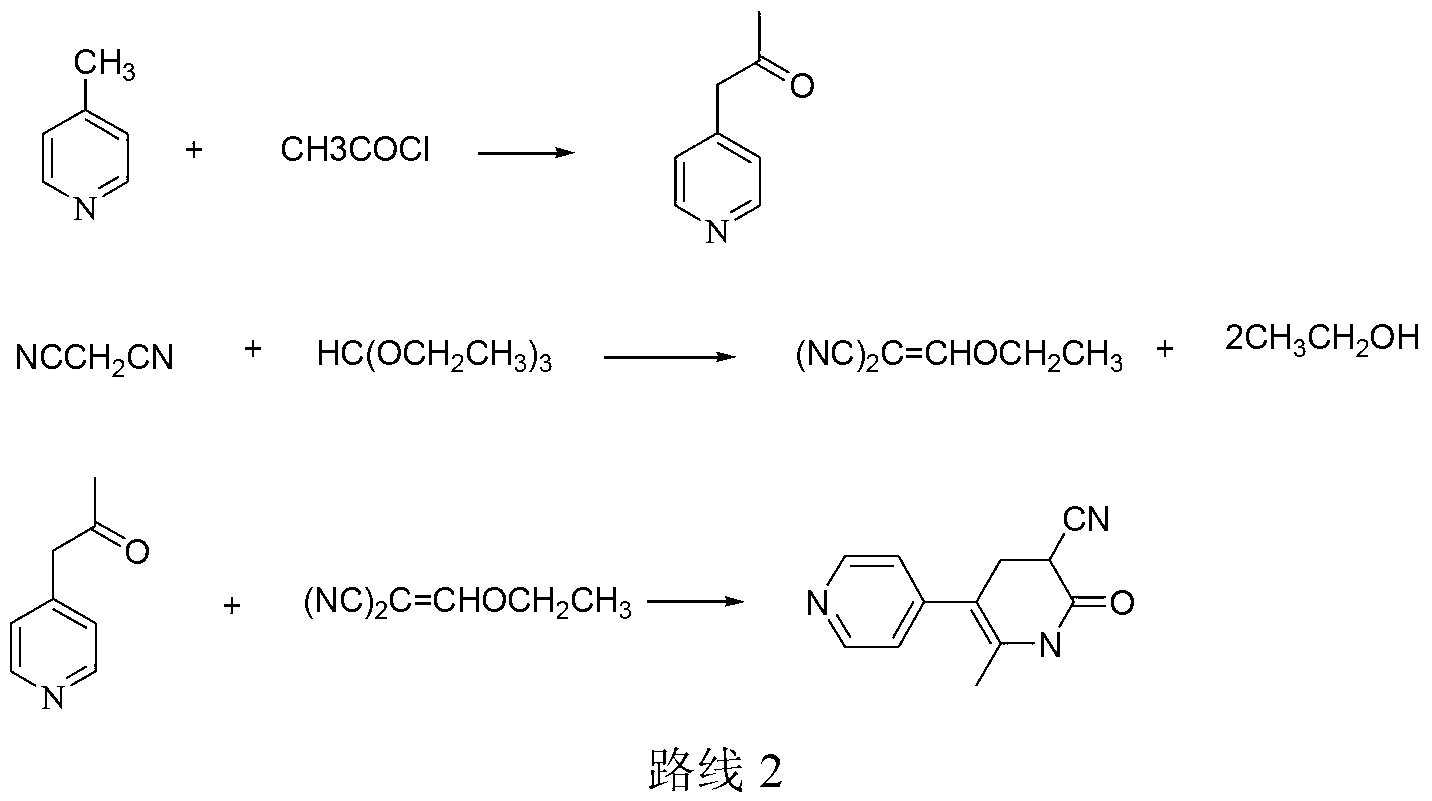
Method for synthesising milrinone
ActiveCN103288725ALow costThe number of times of extraction and decompression concentration is reducedOrganic chemistryAcetic anhydrideSodium bisulfate
The invention discloses a method for synthesising milrinone. The method comprises the following steps of: mixing 4-methylpyridine with acetylchloride in a solvent at a temperature below 10 DEG C; heating to react with or without a catalyst, and then adjusting the pH value of the reaction solution to 7-8 by sodium hydroxide aqueous solution; then directly adding saturated sodium hydrogen sulfite aqueous solution, and reacting; adjusting the pH value of the water layer obtained by the reaction by sodium hydroxide, and reacting to obtain a compound in formula (III); mixing the compound in formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and then reacting at 50-100 DEG C to obtain a compound in formula (IV); cyclizing the compound in formula (IV) with alpha-cyanoacetamide in an alkaline condition to obtain a compound in formula (V). The process is more moderate in reaction conditions, simpler and more convenient to operate, and capable of greatly shortening the original reaction time, reducing cost and increasing yield simultaneously; the process is a preparation method for milrinone which is suitable for industrialized production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Preparation method for high-purity milrinone
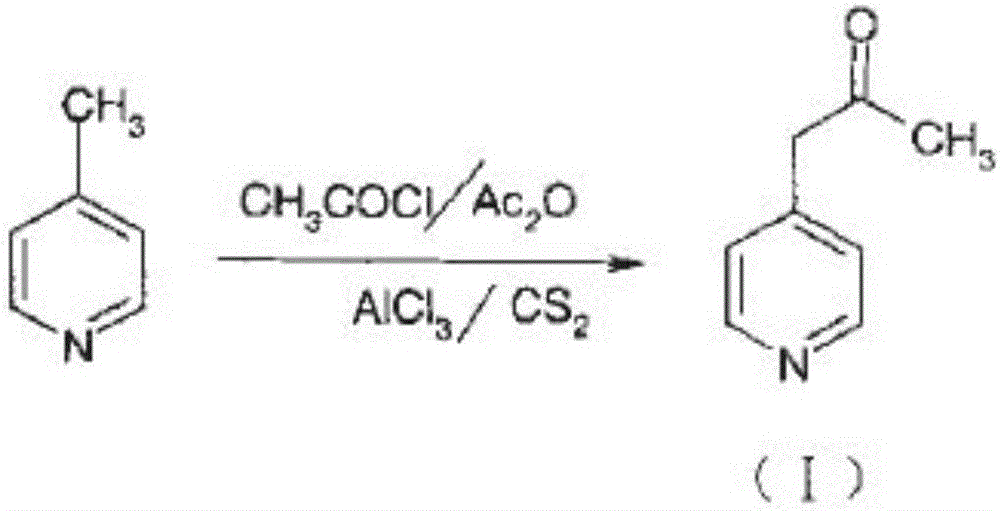
ActiveCN104387320AQuality improvementImprove crystal effectOrganic chemistryAcetic anhydrideSingle crystal
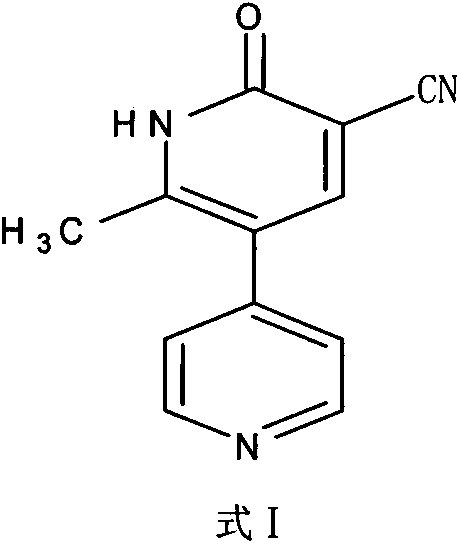
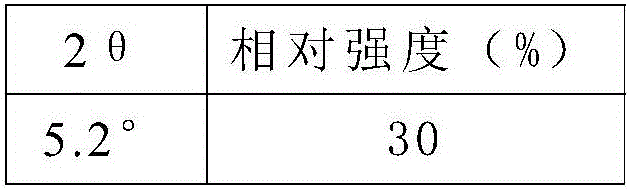
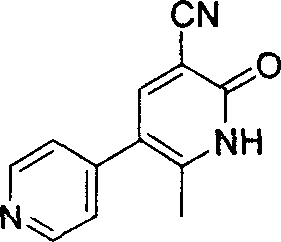
The invention discloses a preparation method for high-purity milrinone (shown as a formula (I), 1,6-dihydro-2-methyl-6-oxo-3,4-bipyridine-5-carbonitrile), and belongs to the field of chemical medicines. The method comprises: employing 4-methylpyridine as a raw material and acetylating with acetyl chloride, and hydrolyzing after the reaction is finished, so as to obtain a compound of a formula (III); mixing the compound of the formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and reacting at 35 DEG C-45 DEG C, so as to obtain a compound of a formula (IV); performing cyclization on the compound of the formula (IV) and alpha-cyanoacetamide, so as to obtain a crude product of a compound of the formula (I); and refining the crude product of the formula (I) compound through an ethanol-water system, so as to obtain a high-purity refined product with the maximum interplanar spacing d of 8.39 + / - 0.02 Angstrom. The technology is relatively mild in reaction conditions and relatively simple in operation, and is capable of preparing the milrinone product with high purity and a single crystal form. The obtained milrinone crystal form is relatively excellent in solubility in normal saline or glucose, and is beneficial for improvement of the preparation quality.
Owner:HUZHOU ZHANWANG PHARMA
Preparing method and refining method for milrinone
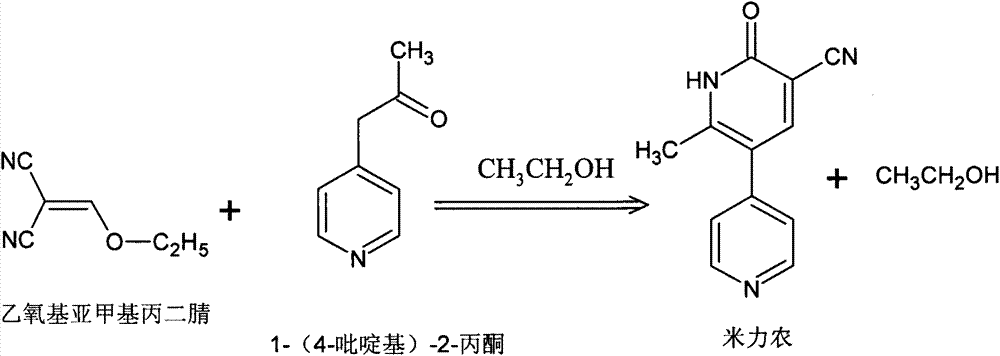
The invention relates to a preparing method and a refining method for milrinone. The preparing method comprises the following steps: an absolute ethyl alcohol solution of 1-(4-pyridyl)-2-acetone is added dropwise into an absolute ethyl alcohol solution of ethoxymethylenemalononitrile, heat preservation is performed for 1-3 h under a condition of 20 DEG C-60 DEG C, then the temperature is increased to 75 DEG C-85 DEG C, a reaction liquid is cooled to 0-50 DEG C after a reaction is completed, solid-liquid separation is performed, and a solid is collected to obtain a product, wherein the mass ratio of ethoxymethylenemalononitrile to 1-(4-pyridyl)-2-acetone is (1.5-3):(1.5-2.5). According to the preparing method and the refining method, water is adopted to replace a purification solvent such as DMF (dimethyl formamide), methanol, ethanol and the like, so that not only are impurities removed effectively, all single impurities in a finished milrinone product is smaller than 0.1%, and solvent residues reach the standard, but also the production cost is reduced, product quality and yield are improved, and the methods are suitable for industrial production.
Owner:南京易亨制药有限公司
Milrinone salt preparation method and its uses
InactiveCN1951919ASolve solubilityFix stability issuesOrganic active ingredientsPowder deliveryMilrinonePotassium hydroxide
The invention discloses a preparing method of Milinong salt and application in the lyophilized product, which is characterized by the following: adopting refined Milinong as raw material; reacting with alcaine, phosphoric acid, sulfuric acid, methanesulfonic acid, sodium hydroxide and potassium hydroxide to obtain the soluble product; blending soluble Milinong salt and auxiliary material to form injection lyophilized product.
Owner:INST OF PHARMACY SHANDONG PROV ACAD OF MEDICAL SCI
Milrinone pharmaceutical composition and preparation method thereof
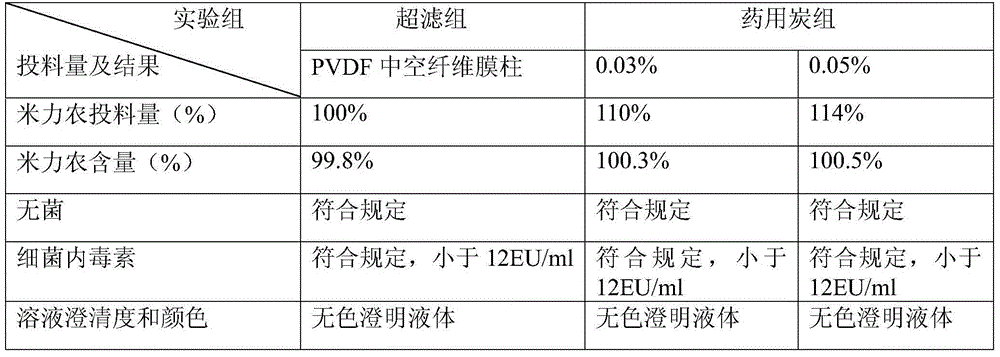
InactiveCN105663034AReduce the amount of feedLarge adsorption capacityOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneFiltration
The invention discloses a milrinone pharmaceutical composition. Either L-lactic acid or L-malic acid is used as a cosolvent and a pH value regulator, and an ultra-filtration method is adopted for filtering. The milrinone pharmaceutical composition provided by the invention can improve hyperchloremia caused by excessive Cl- and the like generated from the adoption of inorganic acid and can improve metabolic disorders and even acidosis caused by D-lactic acid or lactic acid. The process for preparing the milrinone pharmaceutical composition provided by the invention is simple to operate and is capable of improving medication safety.
Owner:YANGZIJIANG PHARMA GROUP SHANGHAI HAINI PHARMA
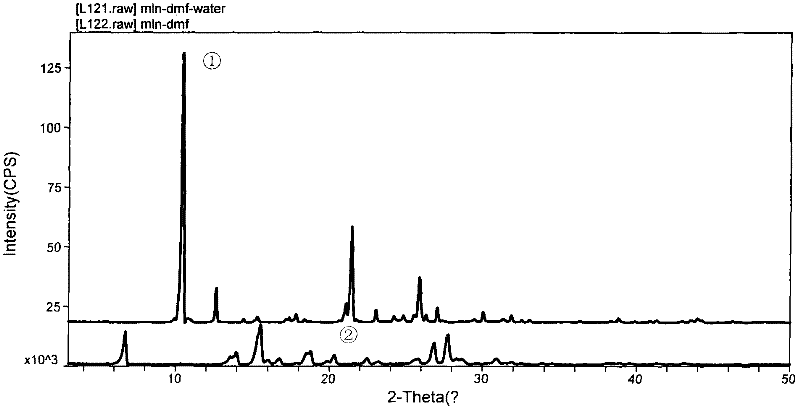
Method for crystallizing milrinone
The invention provides a method for crystallizing milrinone without recrystallizing. A mixed solvent is used, and hot water is added to dimethyl formamide (DFM), thus the defects of multiple re-crystallization low crystal purity due to the utilization of signal solvent in the prior art are overcome. In the last step of subjecting materials to reaction to synthesize the milrinone, the crystallization is carried out to obtain the milrinone with a good crystal form, higher purity and higher yield, thus the operation and the reaction process are simplified, the pollution is reduced, and the cost is lowered. The milrinone is suitable for large-scale industrial production as the active pharmaceutical ingredient and meets the requirement of the injection preparation.
Owner:方晏燕
Process for preparing milrinone
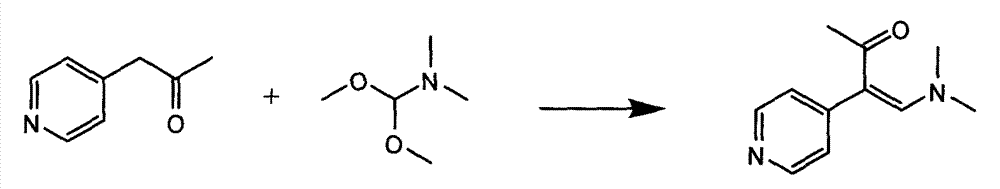
In accordance with the invention, 1-(4-pyridinyl)-propanone is used as raw material for condensation reaction with triethyl orthoformate, wherein the condensate directly reacts with the alpha-cyanoacetamide to obtain Milrinone without the need of purification. The prepared Milrinone is an important chemical product which can be used for preparing cardiotonic drugs.
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of method of synthesizing milrinone
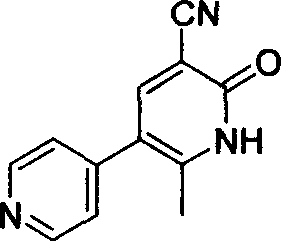
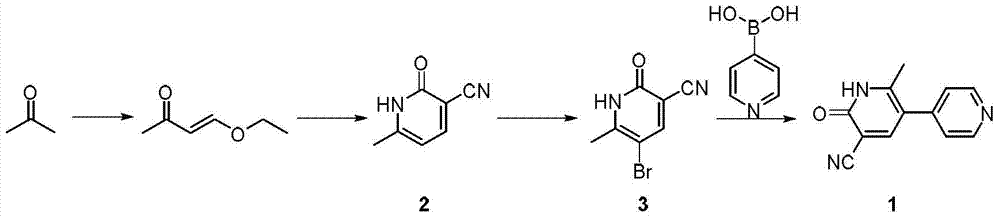
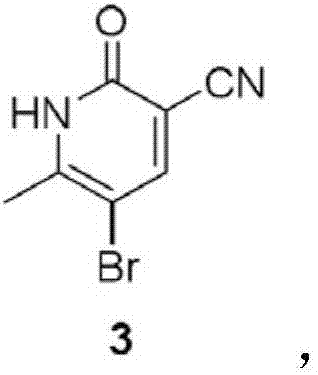
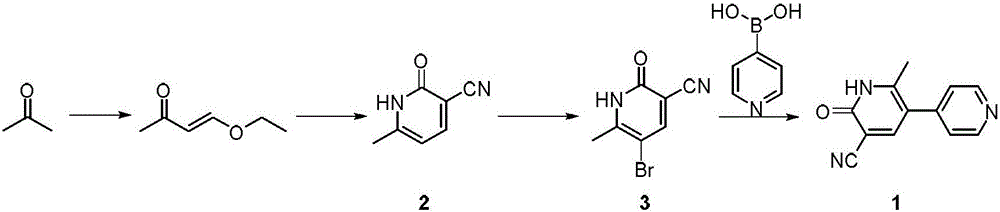
The invention discloses a new method for synthesizing milrinone. The method is characterized in that a compound of formula 3 and 4-pyridineboronic acid undergo a Suzuki coupling reaction to synthesize milrinone. The method has the advantages of easily available raw materials, high yield and simple post-treatment.
Owner:HUNAN SAILONG PHARMA
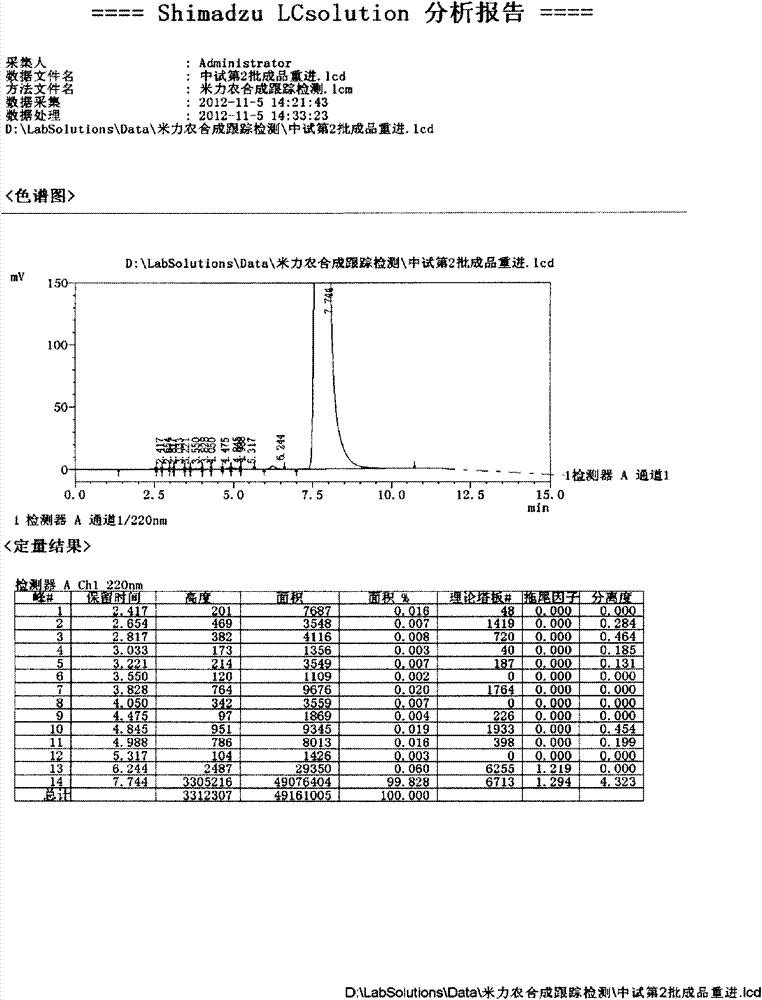
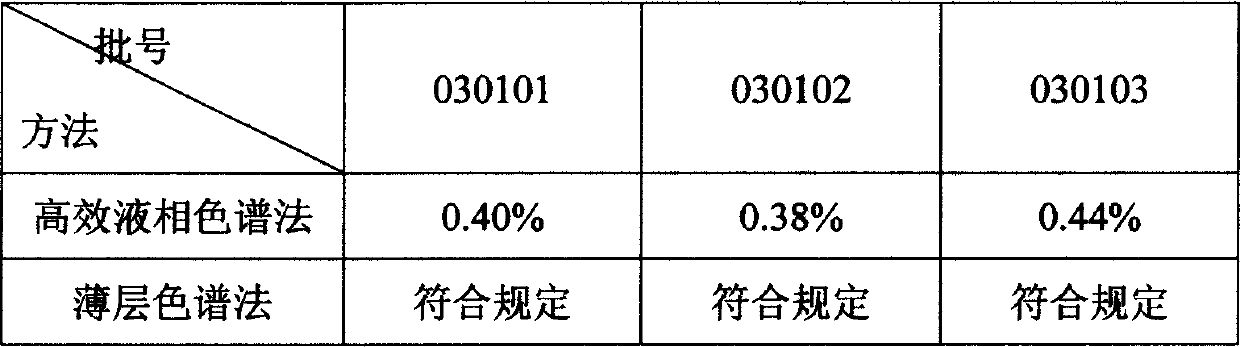
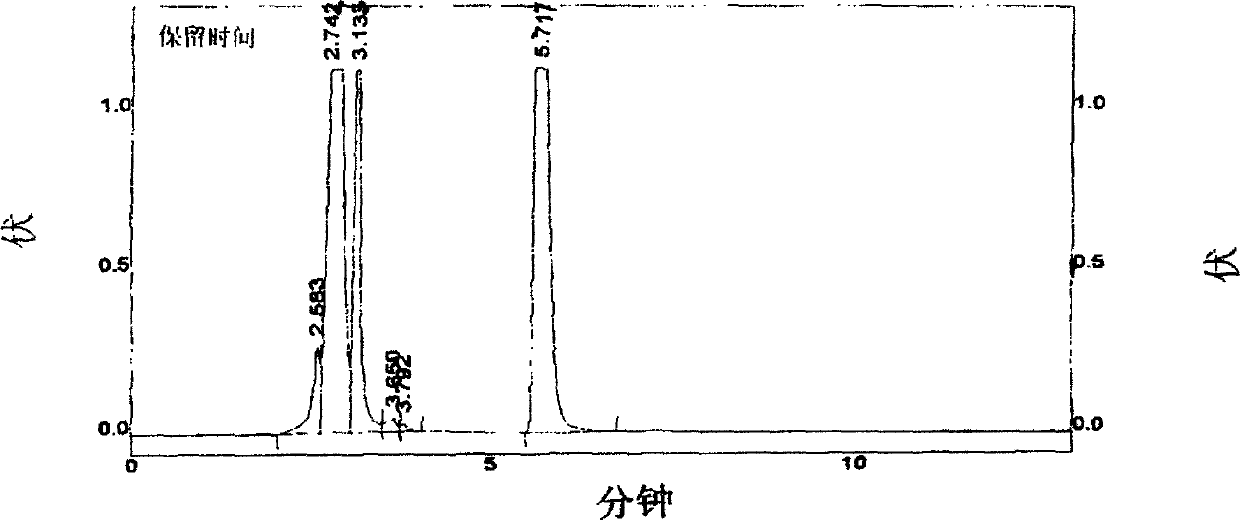
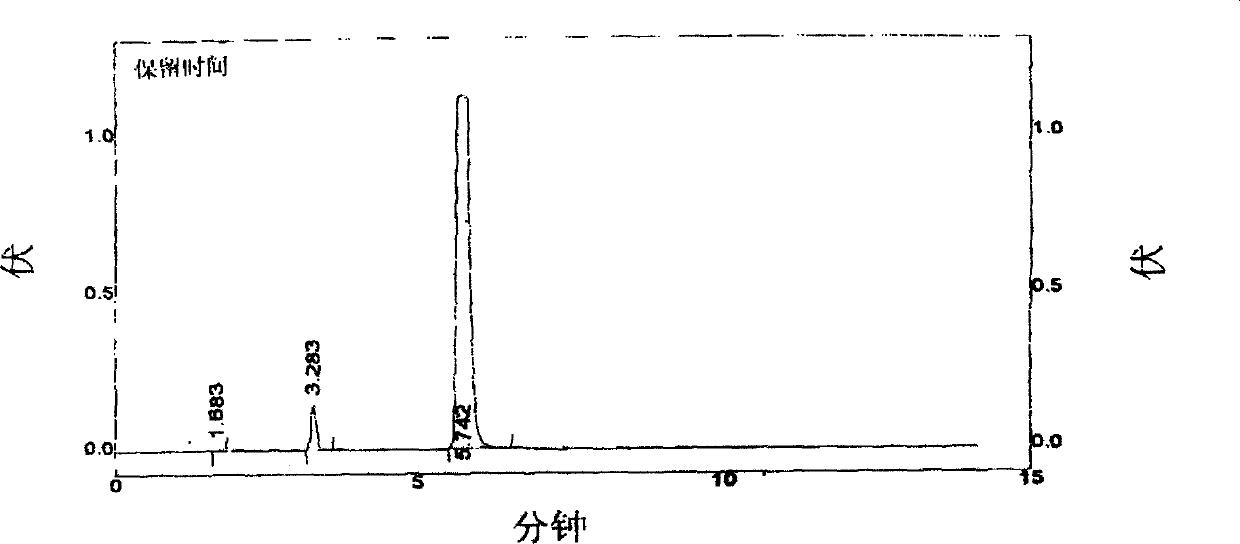
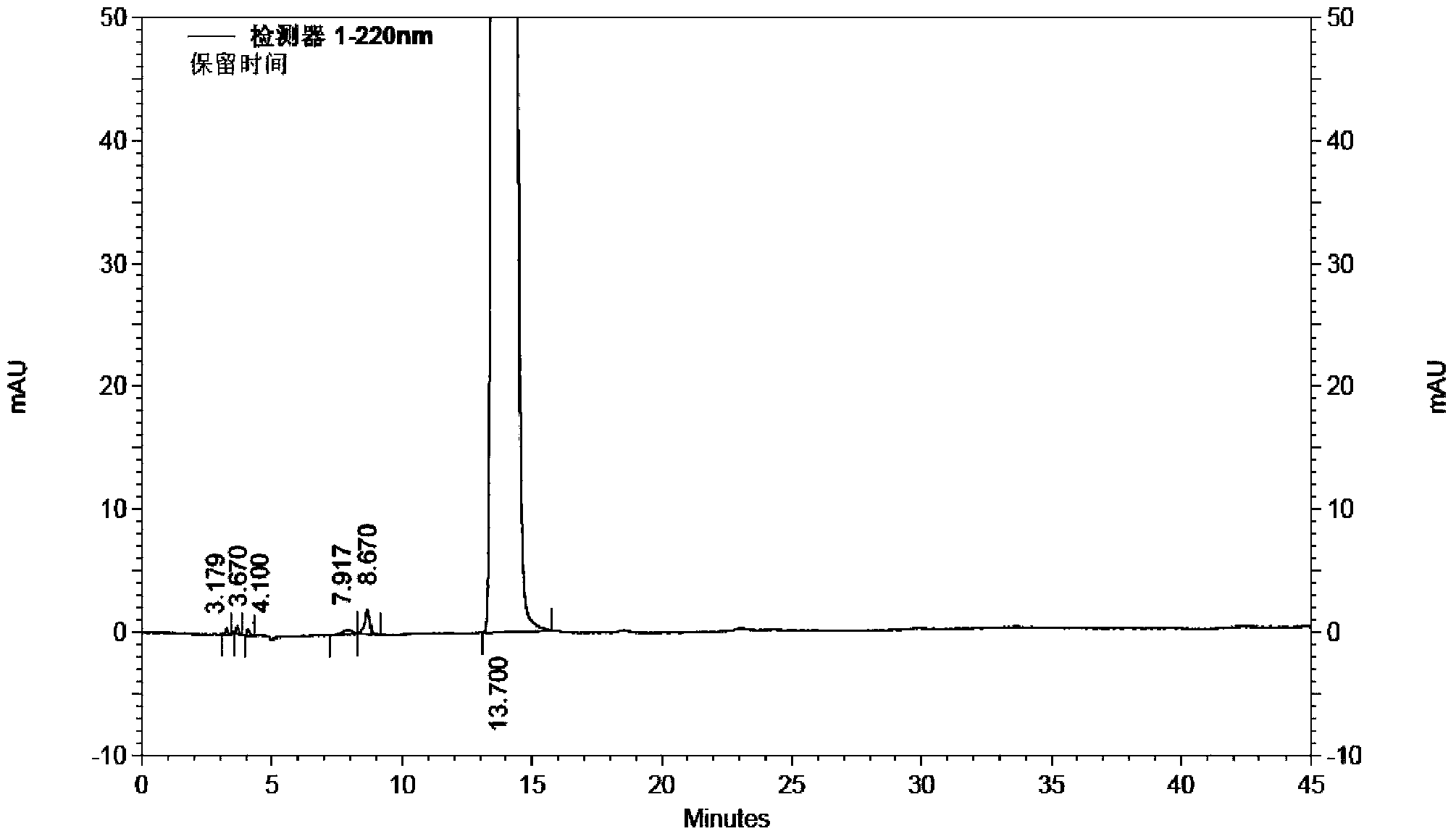
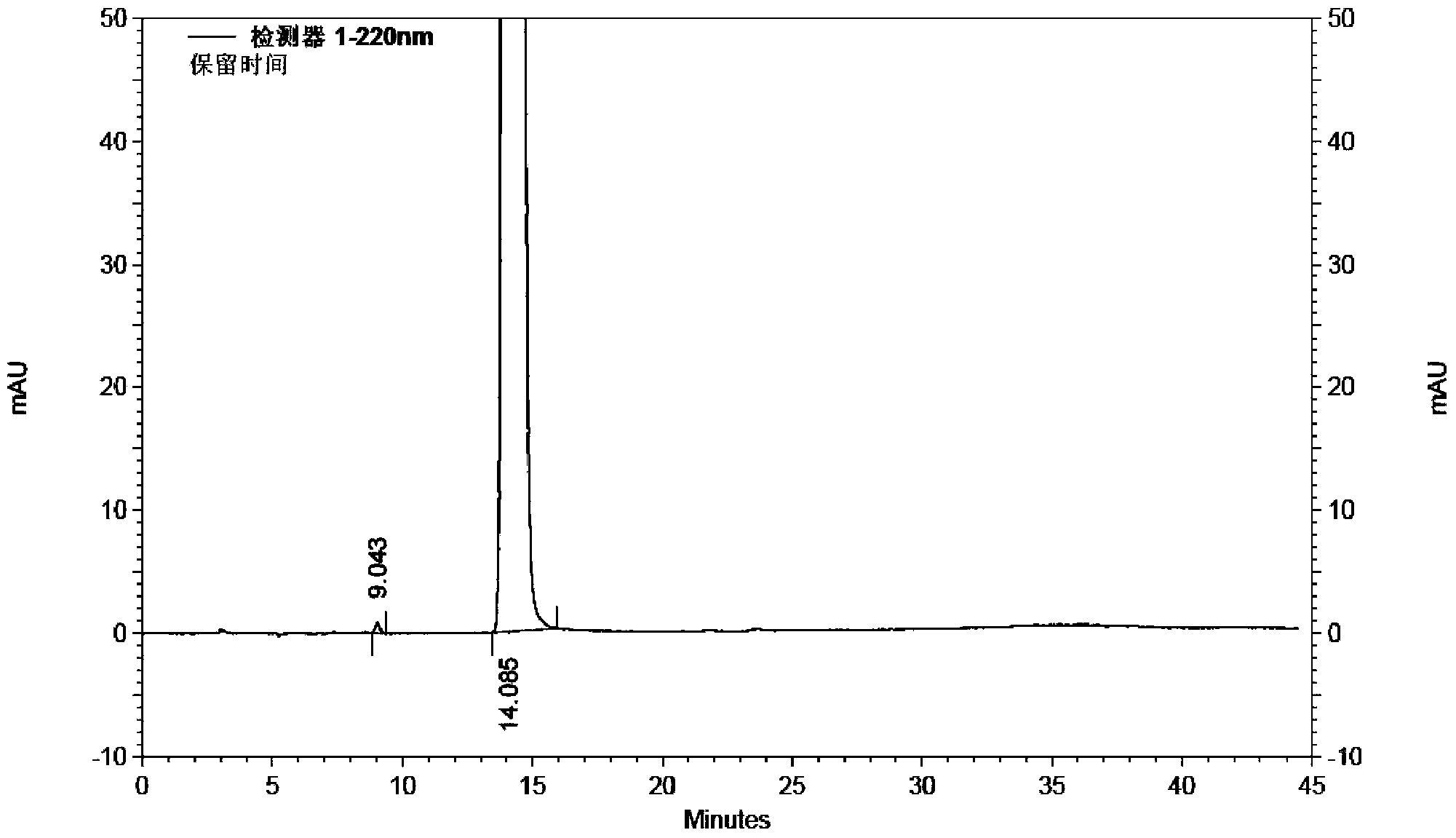
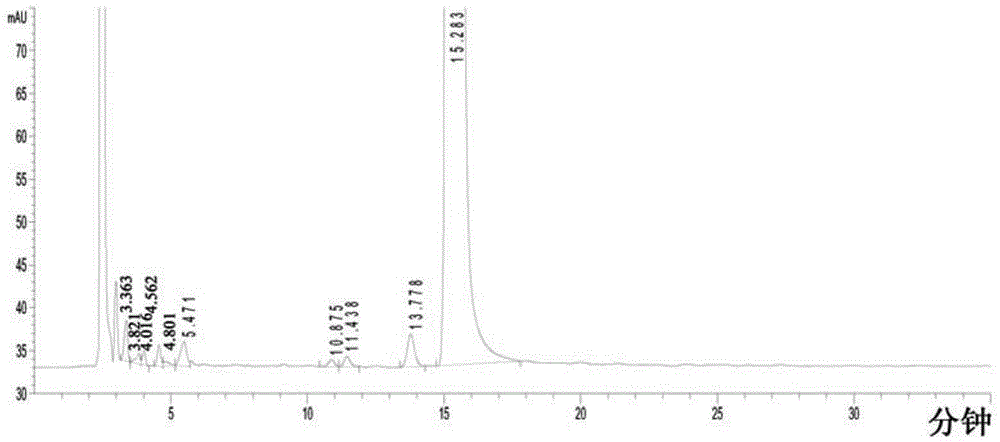
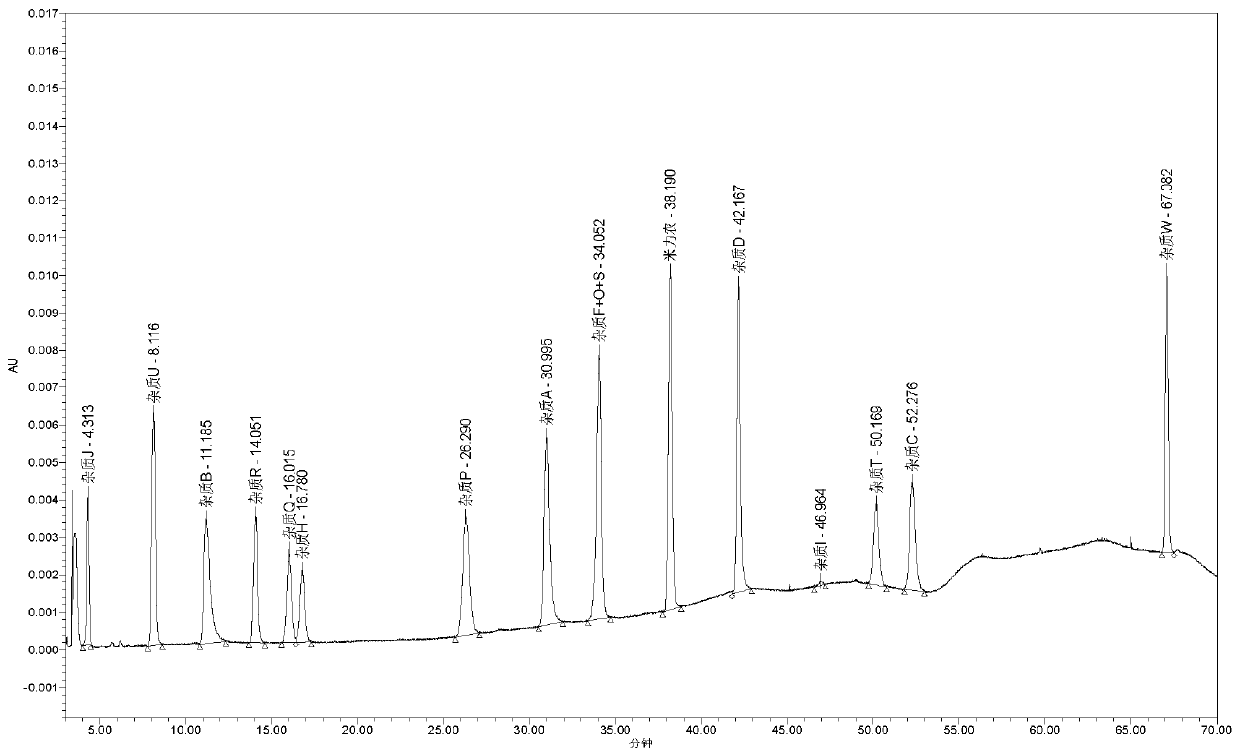
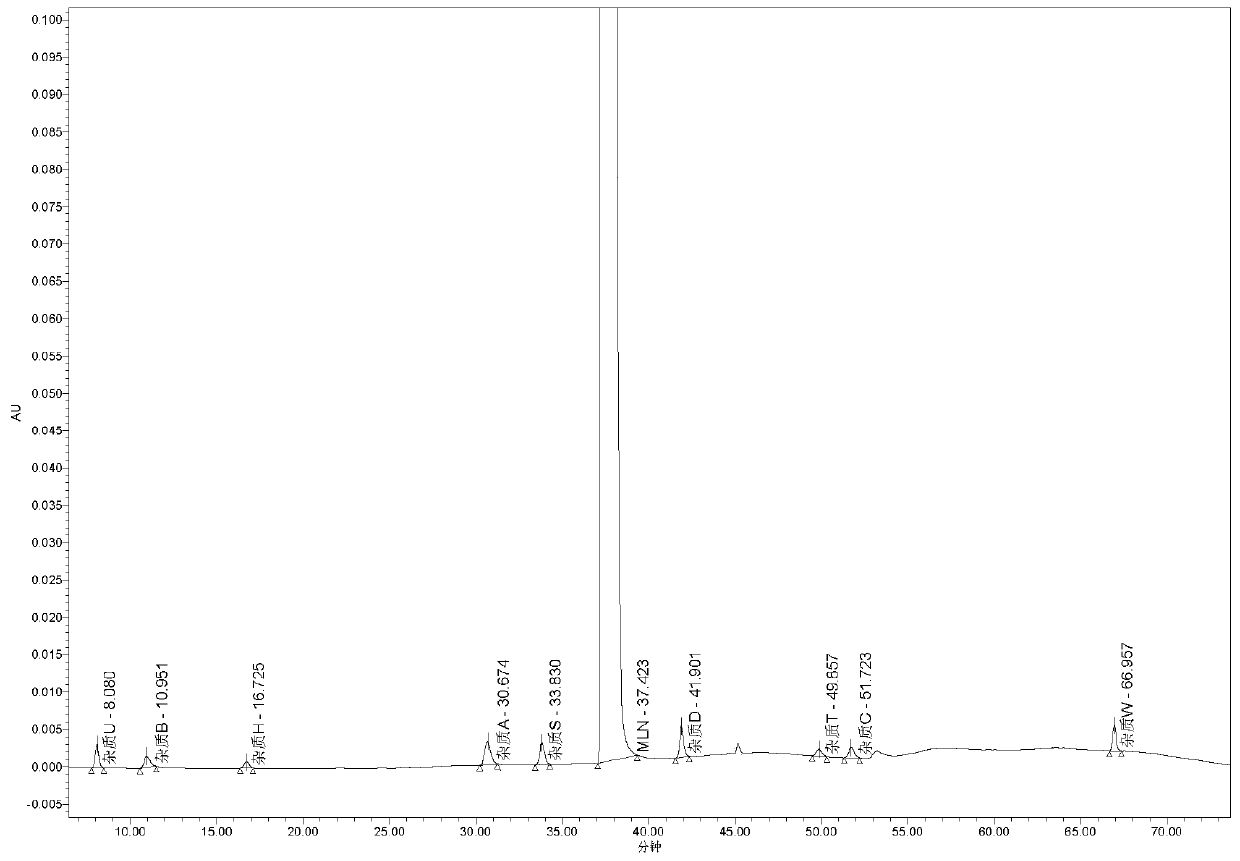
Use of high efficiency liquid chromatography in measuring content of related substances in Milrinone
The present invention provides the application of high efficiency liquid phase chromatographic process in measuring the relevant matters and their content in milrinone. The flowing phase may consist of borax buffering liquid, methanol and water in the proportion of 1-5 to 20-50 to 50-80, preferably in the proportion of 1-3 to 20-40 to 60-70, and the borax buffering liquid has the preferable concentration of 0.05 M and pH value of 7.0.
Owner:LUNAN PHARMA GROUP CORPORATION
Milrinone lyophilized powder for injection and preparation method thereof
InactiveCN1879622AFrozen crystals are fineDissolve fastPowder deliveryOrganic active ingredientsMilrinonePharmacology
The invention relates to an injection Milrinone freeze powder and relative preparation, which is characterized in that: it comprises active Milrinone and pH adjuster; the content of pH adjuster can make the pH value before freezing the powder at 2.5-3.0. The invention can make frozen product with fine crystal, and quick soluble speed, with uniform component and better stability.
Owner:SHANGHAI SINE PHARMA LAB
Method and substance for facilitating weaning, reducing morbidity and reducing mortality in cardiac surgeries involving extra-corporal circulation
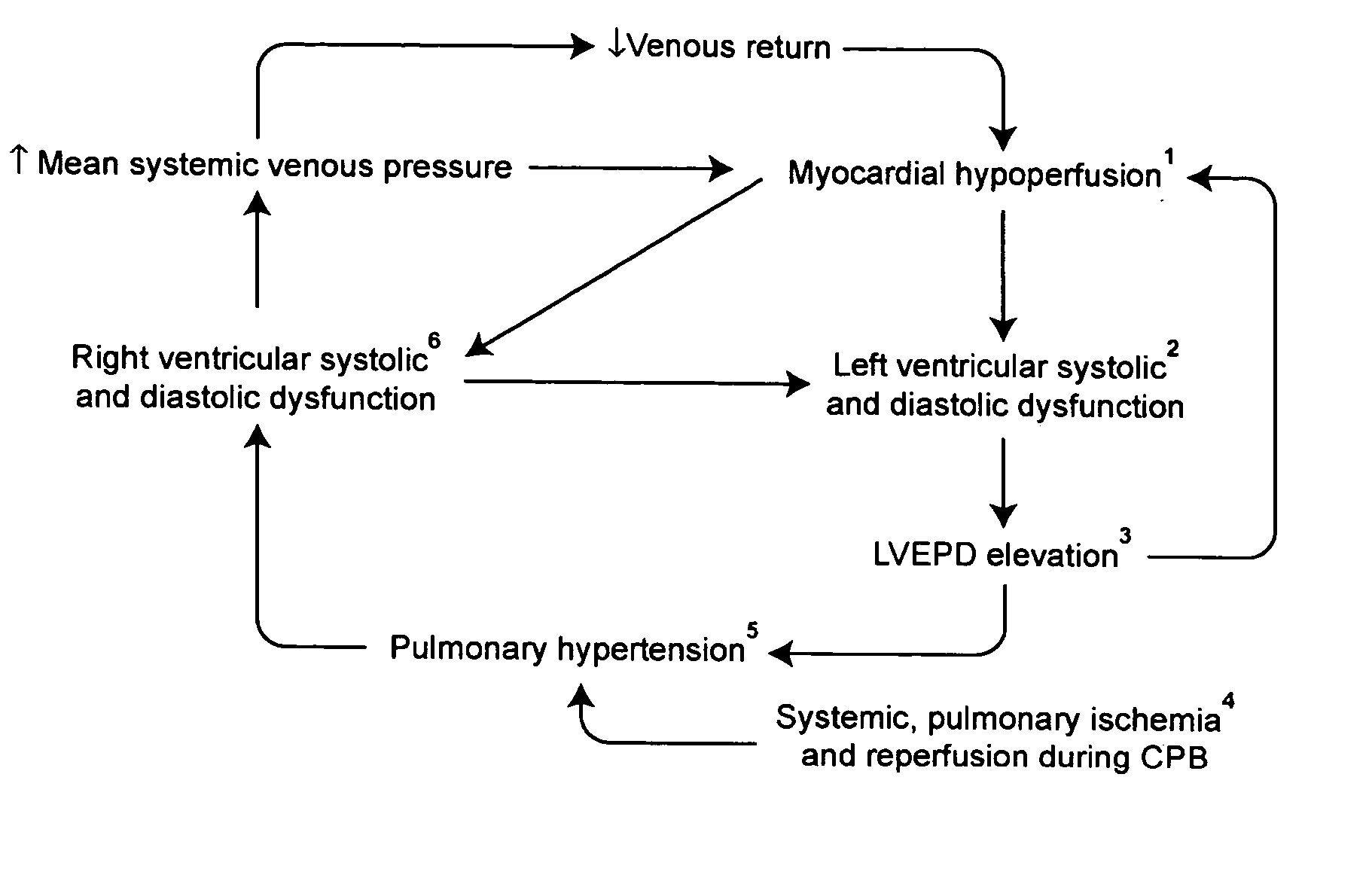
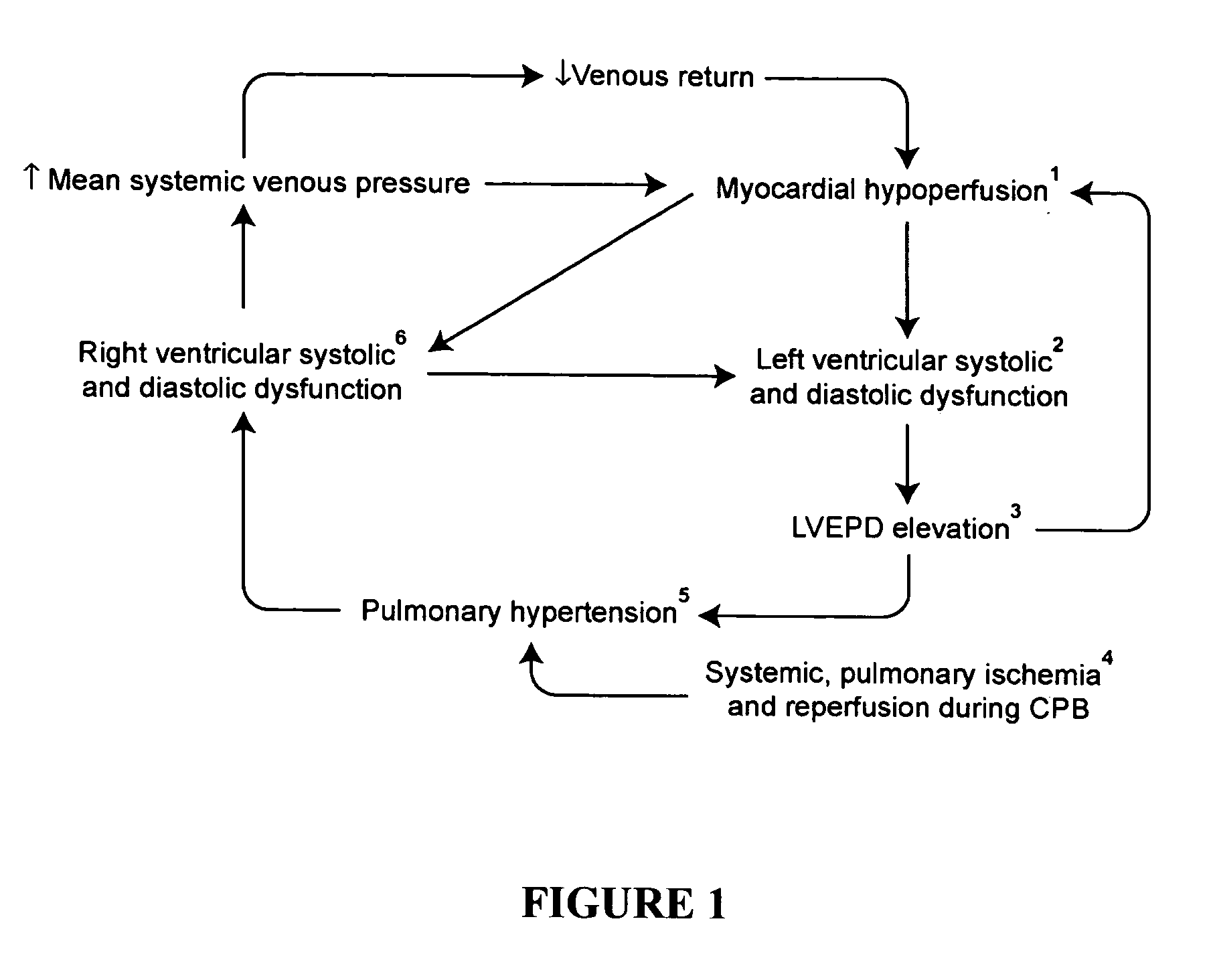
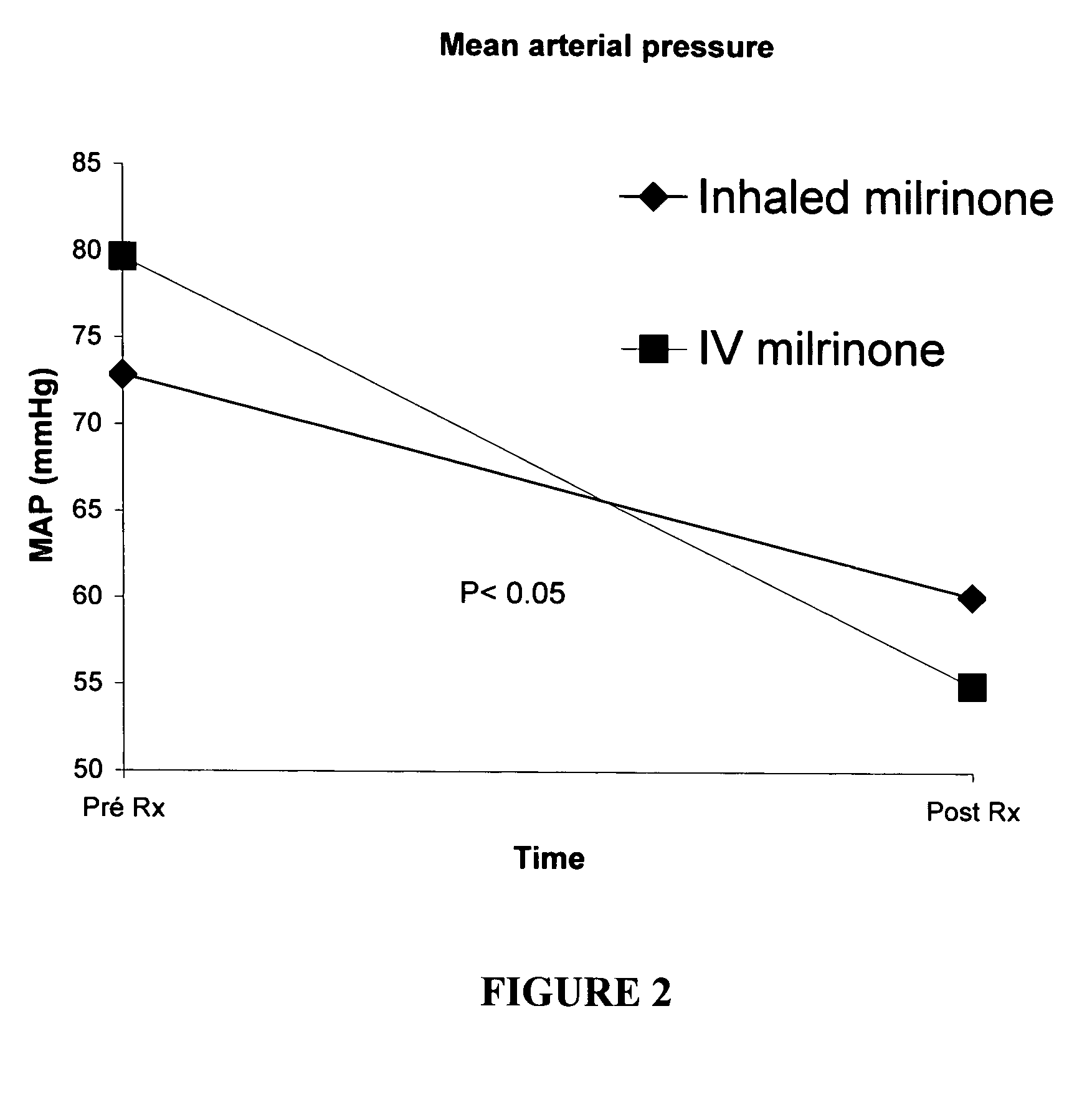
InactiveUS20050049174A1Prevent right dysfunctionReducing right ventricular afterloadOrganic active ingredientsBiocideDobutamineVascular dilatation
Prophylactic strategies aimed at delivering vasodilators through inhalation in the pulmonary tree treat and prevent right ventricular dysfunction by reducing right ventricular afterload, facilitate separation from bypass and consequently decrease hemodynamic complications, morbidity and mortality. Examples of suitable vasodilatator include prostacyclin (flolan®), amrinone (inocor®), dobutamine (dobutrex®), nitroglycerine, nitroprussiate (nipruss®) and milrinone (primacor®).
Owner:INST DE CARDIOLOGIE DE MONTREAL
Recrystallization purification method of milrinone
InactiveCN104744357AReduce dosageHigh crystallinityOrganic chemistry methodsMilrinoneActivated carbon
The invention relates to a recrystallization purification method of milrinone, belonging to the field of active pharmaceutical ingredient refinement. The method comprises the following steps: adding a mixed solvent into a milrinone crude product, slowly heating, dissolving, adding activated carbon for decolorization, and carrying out vacuum filtration while the solution is hot to remove the activated carbon; cooling the filtrate to room temperature while slowly stirring, crystallizing, carrying out vacuum filtration, washing the filter cake with ethanol, and drying to obtain a white-like crystal; and adding the mixed solvent into the white-like crystal, slowly heating until all the white-like crystal is dissolved, cooling to room temperature while slowly stirring, and crystallizing to obtain a white crystal which is the purified milrinone with the content of up to 99.90%. The mixed solvent composed of water, ethanol and DMF (N,N-dimethylformamide) is utilized for filtration and purification, thereby comprehensively satisfying the requirements for solvent consumption, crystallinity and content. The product provided by the invention has obviously better appearance (such as crystallinity and color) than the product in the prior art.
Owner:美信佳中维药业股份有限公司
Milrinone composition for injection and its prepn
InactiveCN1739512AHigh concentration of milrinoneOrganic active ingredientsPowder deliveryHigh concentrationMilrinone
The present invention provides one kind of freeze dried high concentration milrinone powder for injection and its preparation process. The freeze dried milrinone powder for injection contains: milrinone in 2-10 weight portions; pharmaceutically acceptable acid matter, which is L-aspartic acid, tartaric acid, citric acid, glutamic acid or their combination, in the amount of 0.25-4 times weight of milrinone; and pharmaceutically acceptable carrier in 15-100 weight portions. The freeze dried milrinone powder for injection may be dissolved in water to form injection of milrinone in 2-10 mg / ml concentration, and has high stability.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD
Synthesis method of milirinone
InactiveCN103804288AEasy to operateReduce pollutionOrganic chemistryChemical industry4-Methylpyridine
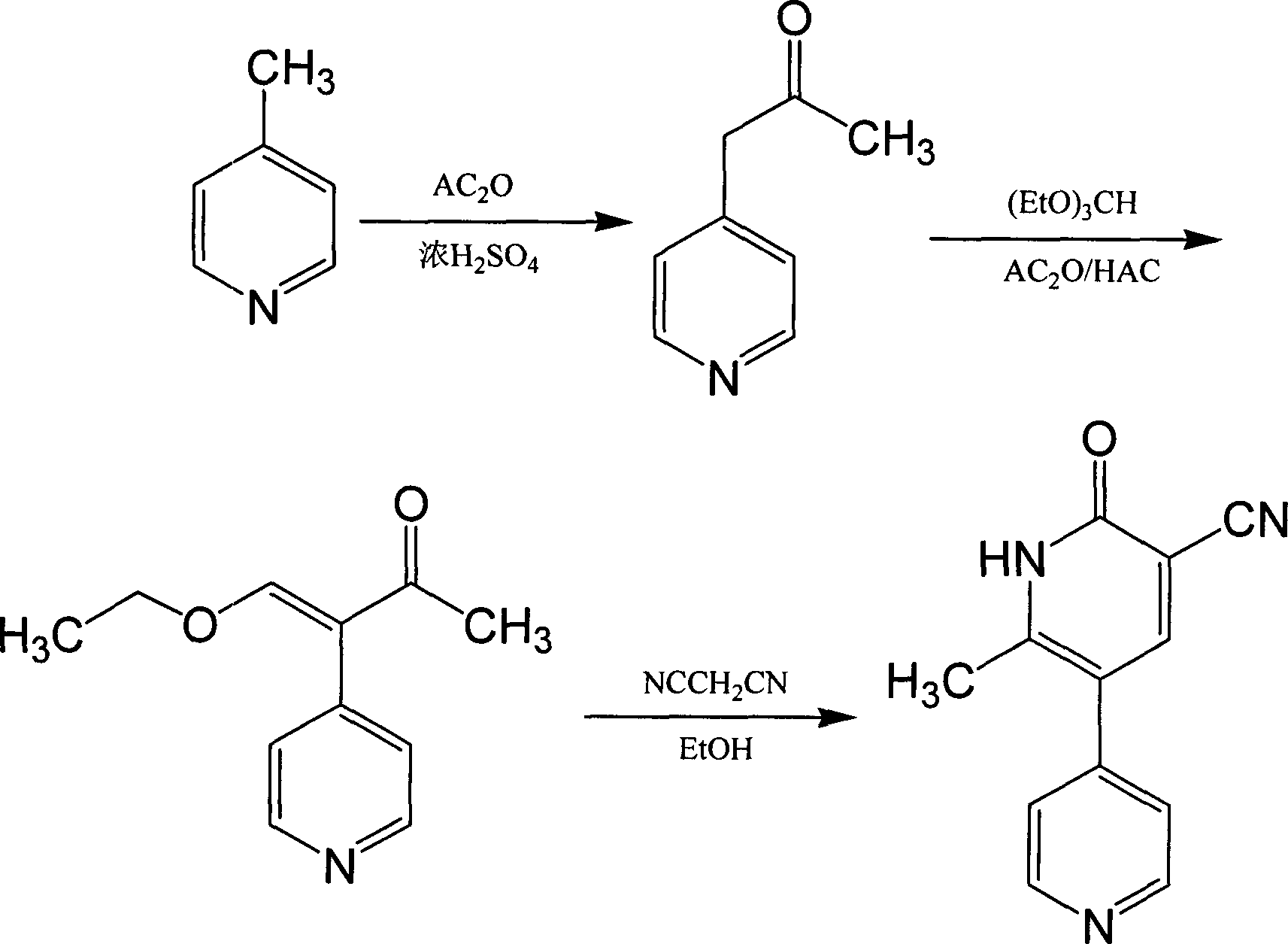
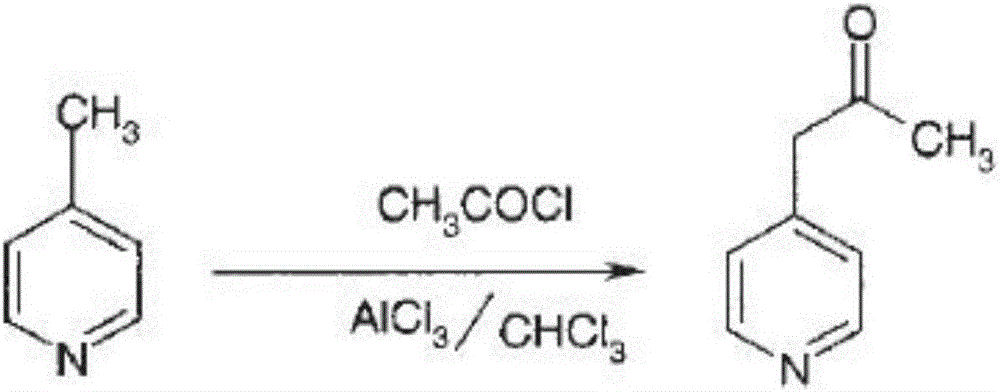
Belonging to the fields of chemical industry and chemical medicine, the invention discloses a synthesis method of milirinone. The method includes: adopting 4-methylpyridine as a raw material to undergo acetylation reaction with acetic anhydride, subjecting the acetylate to condensation with triethyl orthoformate directly without purification, and subjecting the condensation product and malononitrile to cyclization reaction in ethanol absolute, thus obtaining milirinone. The method is characterized by starting with relatively simple and easily available raw materials to directly obtain a complicated structure molecule without separation and purification of the intermediates. The milirinone synthesized by the method provided by the invention has the advantages of high yield and high sample purity. Milirinone is an important chemical product, and can be used as a cardiotonic drug and the like.
Owner:SICHUAN XINSIDUN PHARMA
Angiotensin receptor antagonist and milrinone compound and use thereof
ActiveCN106237334AGood treatment effectConvenient treatmentMetabolism disorderGranular deliveryMilrinoneHydrogen
The invention provides an angiotensin receptor antagonist and milrinone compound and a use thereof. The compound comprises an angiotensin receptor antagonist and milrinone, the angiotensin receptor antagonist and milrinone can be directly mixed, or can be indirectly connected through hydrogen bonds, and cocrystallized sodium hydrate formed by using the hydrogen bonds have stable properties and substantially improved pharmacokinetic properties. The action mechanisms of the angiotensin receptor antagonist and milrinone are different, but the compound formed by the angiotensin receptor antagonist and milrinone have unexpected synergism, so the compound has positive application prospect in the anti-heart failure and anti-hypertension field.
Owner:赛隆药业集团股份有限公司(长沙)医药研发中心
Method for preparing milrinone
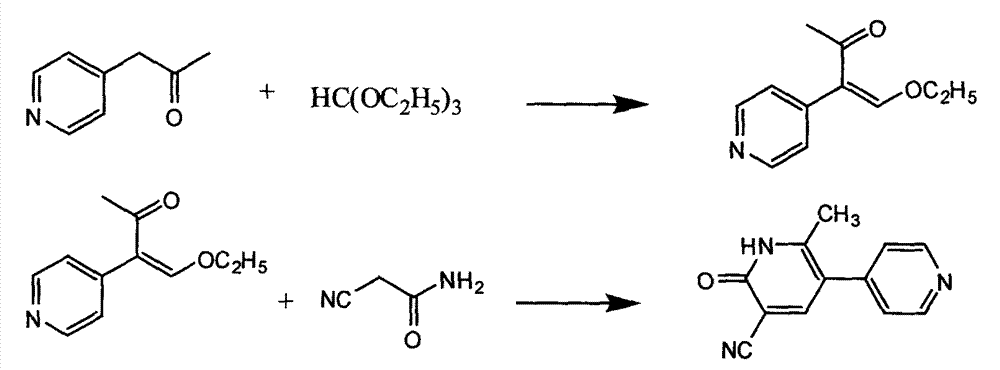
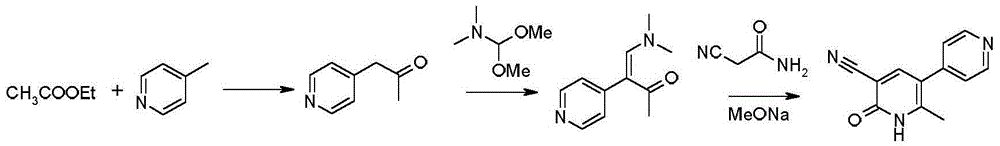
The invention provides a method for preparing milrinone, wherein the method comprises the following steps: with 4-methyl pyridine (SM) as a raw material, in ethyl acetate, generating 1-(4-pyridyl)-2-acetone (represented by the formula I); then under action of triethyl orthoformate, acetic acid and acetic anhydride, generating 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone (represented by the formula II); and finally, under an alkaline condition, carrying out a reaction of 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone with cyanoacetamide to generate milrinone. The method has the advantages of simple and efficient operation, mild reaction conditions, strong safety, easy control and relatively high yield, and is suitable for industrialized production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Preparation method of high-purity milrinone
InactiveCN104326975AMild preparation conditionsSimple and fast operationOrganic chemistryDimethyl acetalMethanol
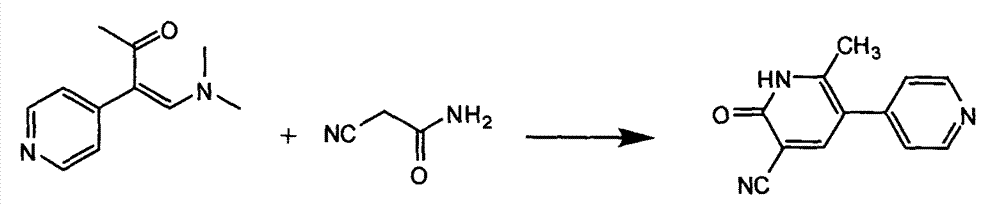
The invention relates to a preparation method of high-purity milrinone as shown in a formula (I) described in the specification. The preparation method comprises the following steps: carrying out reflux reaction on N, N-methyl formamide dimethyl acetal (DMF-DMA) by taking 1-(4-pyridyl) acetone as a starting material, concentrating after the reaction is ended, adding normal hexane into residues to separate out solids to obtain an intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone; carrying out reflux reaction on the intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone and cyanoacetamide in the presence of sodium methylate, and regulating the pH value after the reaction is ended to obtain crude milrinone; and refining the crude milrinone by methanol / sodium methylate and alcohol / water to obtain a qualified milrinone product. The preparation method disclosed by the invention is simple in process operation, relatively small in pollution and suitable for industrial production.
Owner:ZHENGZHOU SIHUAN MEDICINE ARTICLE CO LTD
Preparation method of high-purity milrinone
ActiveCN103965101ALess residueEasy to operate the machineOrganic chemistryMilrinoneMethylvinyl ketone
The invention discloses a preparation method of high-purity milrinone. The method comprises the steps of dissolving 1-ethoxyl-2-(4-pyridyl) methyl vinyl ketone, cyanoacetamide and alkali into a monohydric alcohol-water system, reacting for 15-18 hours at -5 DEG C to 10 DEG C, adding water into reaction liquid and diluting to 1.5 times of the original volume after completing reaction, then adding activated carbon, stirring for 15-25 minutes at room temperature, filtering, regulating the pH value of filtrate to be 7 by using a hydrochloric acid solution, filtering, washing a filter cake by using water until the filtrate is colorless, refluxing and dissolving the filter cake by using 50vt% ethanol, filtering, and stirring and crystallizing the filtrate at -5 DEG C to 10 DEG C to obtain white solid milrinone with the purity of more than 99.9%. The method disclosed by the invention is simple and fast in process, high in yield and pure in product, and is more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
Milrinone sodium chloride injection and production thereof
ActiveCN100337629CImprove toleranceSimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneSodium Chloride Injection
A milrinone-sodium chloride injection for treating congestive heart failure is proportionally prepared from milrinone, sodium chloride and lactic acid.
Owner:LUNAN BETTER PHARMA
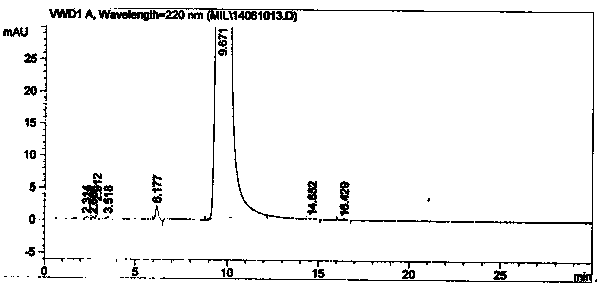
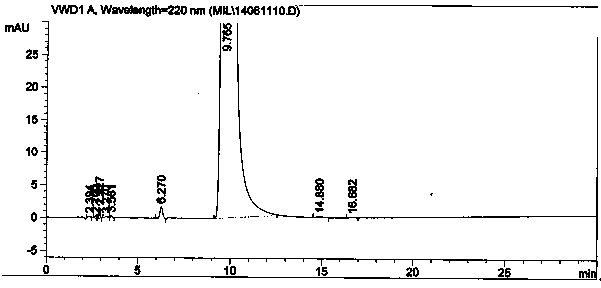
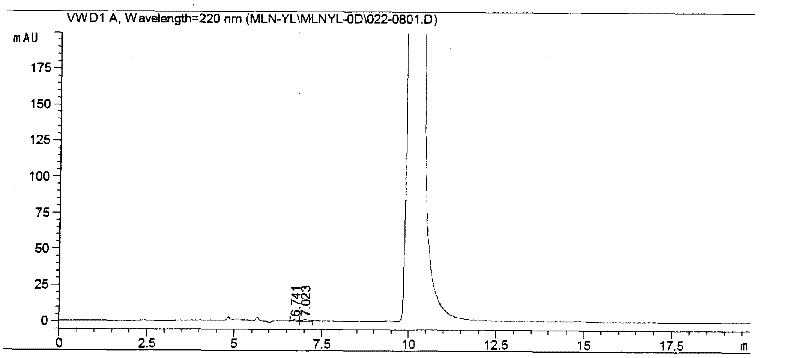
Method for separating and analyzing milrinone
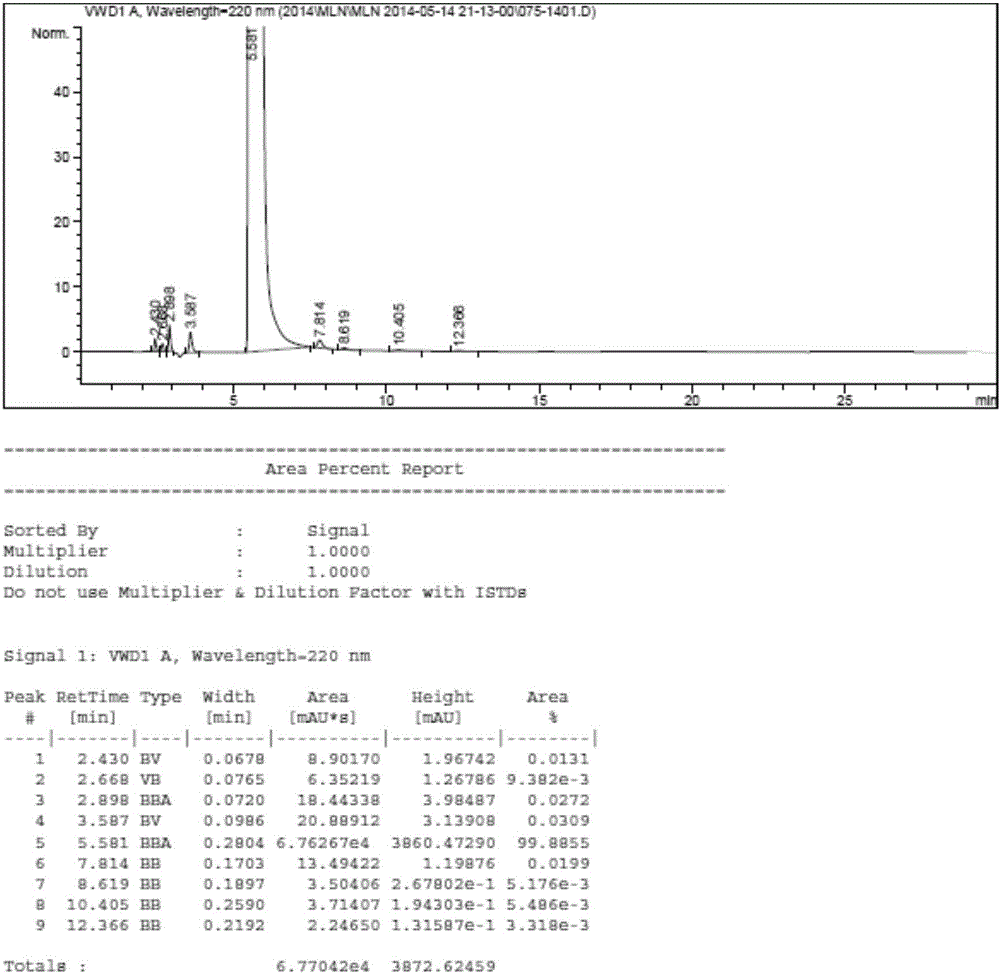
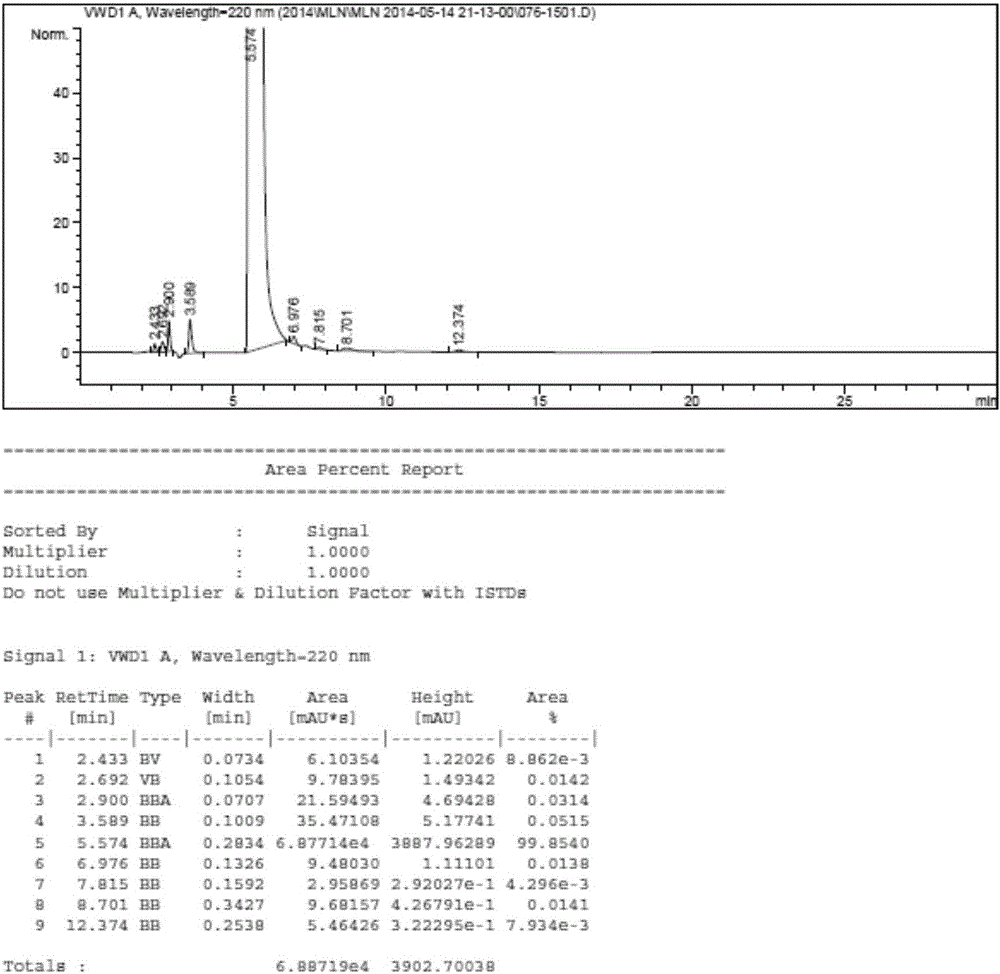
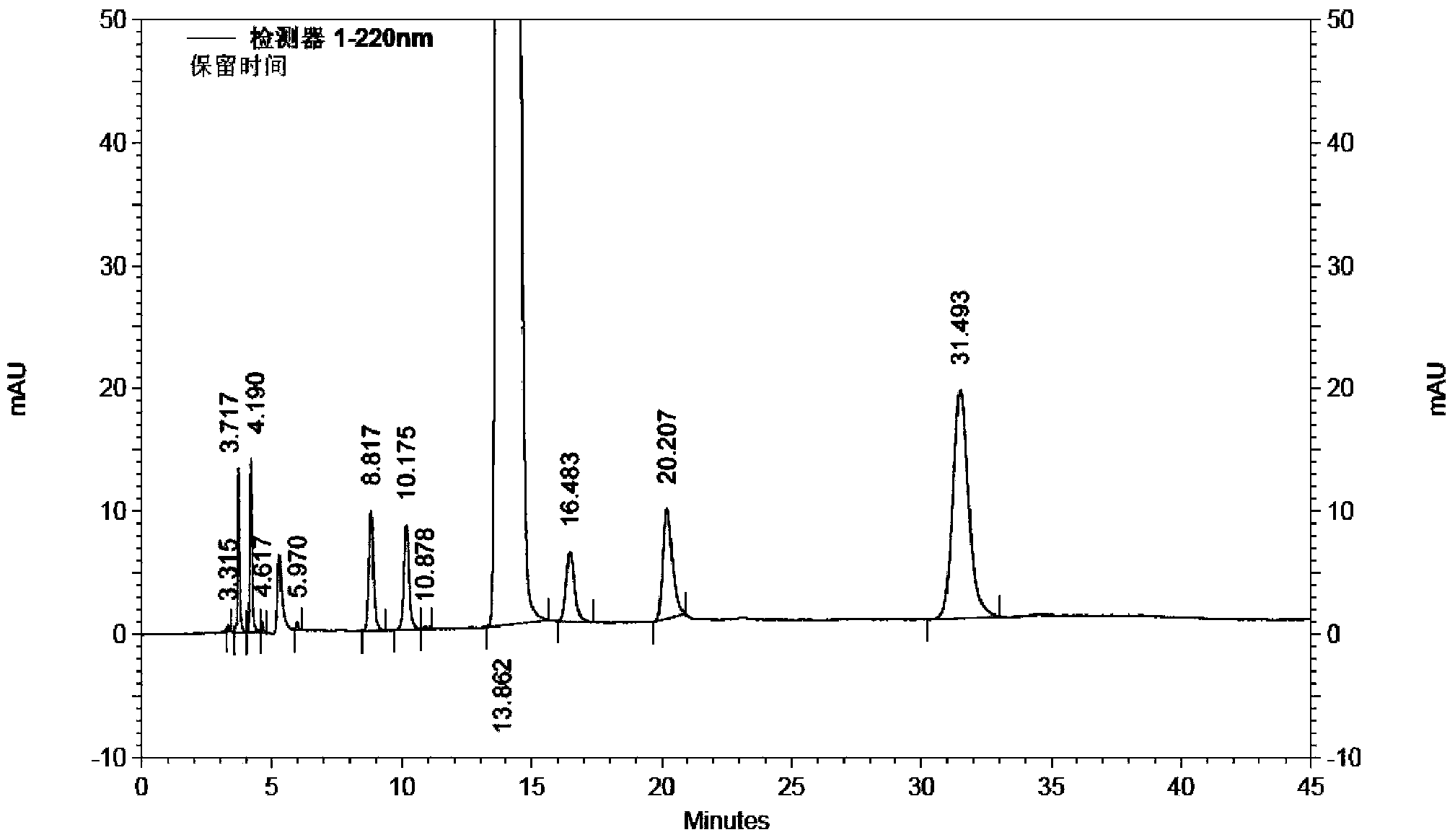
The invention relates to a method for separating and analyzing milrinone. According to the method, a milrinone sample is detected by adopting a high performance liquid chromatography, wherein the high performance liquid chromatography takes a methanol-phosphate solution as a mobile phase, the phosphate solution contains diethylamine or triethylamine, and the pH of the phosphate solution is 5.5-6.5. According to the method provided by the invention, the milrinone can be effectively separated from a synthesized intermediate body-1-(4-pyridyl)acetone and other related substances; the number of theoretical plates of a chromatographic peak is high and a base line is stable; and a relatively large quantity of impurities can be detected so that the sensitivity and the accuracy for detecting the milrinone and the related substances of the milrinone are greatly improved. The method provided by the invention lays a foundation for the quality control of the milrinone and related preparations of the milrinone.
Owner:NEW FOUNDER HLDG DEV LLC +2
Method for preparing milrinone
The invention discloses a method for preparing milrinone. The method comprises the following steps: (1) mixing 4-methyl pyridine and maleic anhydride in a solvent, adding a catalyst, maintaining the temperature to be 30-45 DEG C, continuously stirring, slowly adding propyl alcohol, reacting for 30-60 minutes, and finally evaporating and extracting so as to obtain 1-(4-pyridyl)-acetone; (2) adding triethyl orthoformate, maleic anhydride and maleic acid into a product prepared in the step (1), maintaining the temperature to be 20-45 DEG C, reacting for 1-3 hours, and evaporating so as to obtain 1-ethyoxyl-2-(4-pyridyl) vinyl methyl ketone; and (3) adding ethanol, acetone and propane dinitrile into the product prepared in the step (2), maintaining the temperature to be 40-65 DEG C, reacting for 1-5 hours so as to obtain crude milrinone, and purifying, thereby obtaining the pure milrinone. According to the method for preparing milrinone provided by the invention, reaction conditions are mild, the yield is high, the cost is low, the operation is easy and convenient and industrialized popularization and application are convenient.
Owner:HUZHOU HENGYUAN BIOCHEM TECH
A kind of preparation method of milrinone
ActiveCN103965101BLess residueEasy to operate the machineOrganic chemistryMethylvinyl ketoneMilrinone
The invention discloses a preparation method of high-purity milrinone. The method comprises the steps of dissolving 1-ethoxyl-2-(4-pyridyl) methyl vinyl ketone, cyanoacetamide and alkali into a monohydric alcohol-water system, reacting for 15-18 hours at -5 DEG C to 10 DEG C, adding water into reaction liquid and diluting to 1.5 times of the original volume after completing reaction, then adding activated carbon, stirring for 15-25 minutes at room temperature, filtering, regulating the pH value of filtrate to be 7 by using a hydrochloric acid solution, filtering, washing a filter cake by using water until the filtrate is colorless, refluxing and dissolving the filter cake by using 50vt% ethanol, filtering, and stirring and crystallizing the filtrate at -5 DEG C to 10 DEG C to obtain white solid milrinone with the purity of more than 99.9%. The method disclosed by the invention is simple and fast in process, high in yield and pure in product, and is more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
Process for preparing milrinone
Owner:LUNAN PHARMA GROUP CORPORATION
A kind of preparation method of high-purity milrinone
ActiveCN104387320BImprove crystal effectQuality improvementOrganic chemistrySolubilityAcetic anhydride
Owner:HUZHOU ZHANWANG PHARMA
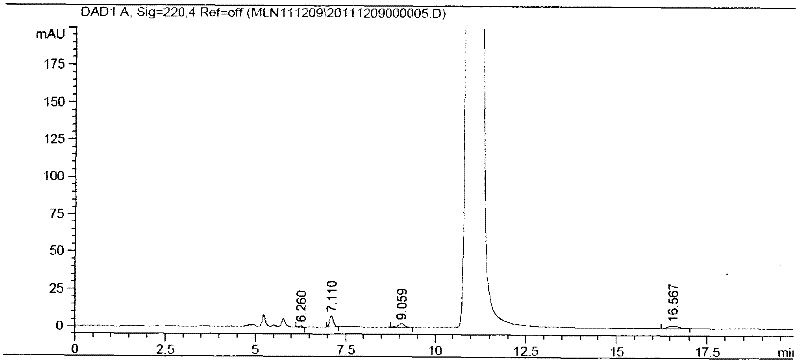
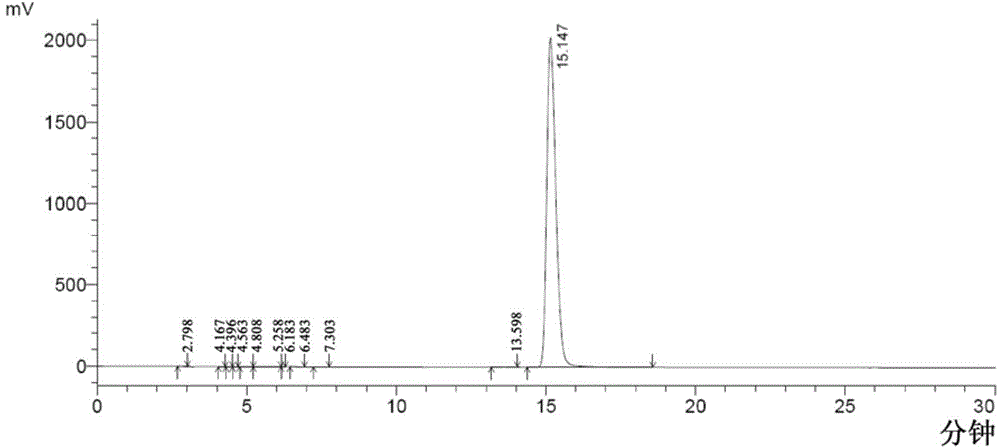
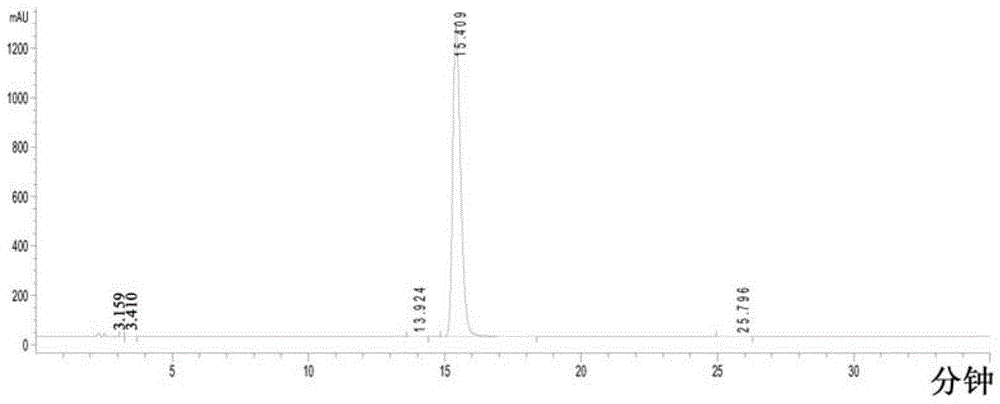
Method for analyzing milrinone related substances by high performance liquid chromatography
The invention discloses a method for analyzing milrinone related substances by high performance liquid chromatography. The method is characterized by adopting a C18 reversed phase chromatographic column, regarding a dipotassium phosphate solution with a pH value ranging from 6.5 to 8.5 as a mobile phase A and regarding water / acetonitrile with a volume ratio of 30:70-60:40 as a mobile phase B for implementing elution, so as to realize detection of milrinog and its 16 kinds of impurities by one sample loading. Compared with the prior art, the method provided by the invention can detect more impurities, and has good sensitivity to the impurities. In the specific application, the method formulates limits of the key impurities according to test results of batches of samples, thereby greatly improving the economic applicability in the actual production process.
Owner:SHANGHAI XUDONG HAIPU PHARMA
Milrinone compound and pharmaceutical composition containing milrinone compound
ActiveCN104173343AAvoid adsorptionGuaranteed sterilization effectOrganic active ingredientsPharmaceutical delivery mechanismMilrinonePh regulation
The invention discloses a milrinone compound and a pharmaceutical composition containing the milrinone compound. According to the invention, lactic acid is adopted for helping solubilization and regulating the pH value, so that hyperchloremia which is possibly caused by extremely high Cl- concentration brought by pH regulation by adopting hydrochloric acid is avoided. In addition, the milrinone compound and the pharmaceutical composition are reasonable in prescription, high in quality standard, low in impurity content and high in bioavailability, has unique advantages in safety, effectiveness and quality stability, and also has an international level and more outstanding advantages.
Owner:朗天药业(湖北)有限公司
New method for synthesizing milrinone
The invention discloses a new method for synthesizing milrinone. The method is characterized in that a compound of formula 3 and 4-pyridineboronic acid undergo a Suzuki coupling reaction to synthesize milrinone. The method has the advantages of easily available raw materials, high yield and simple post-treatment.
Owner:HUNAN SAILONG PHARMA
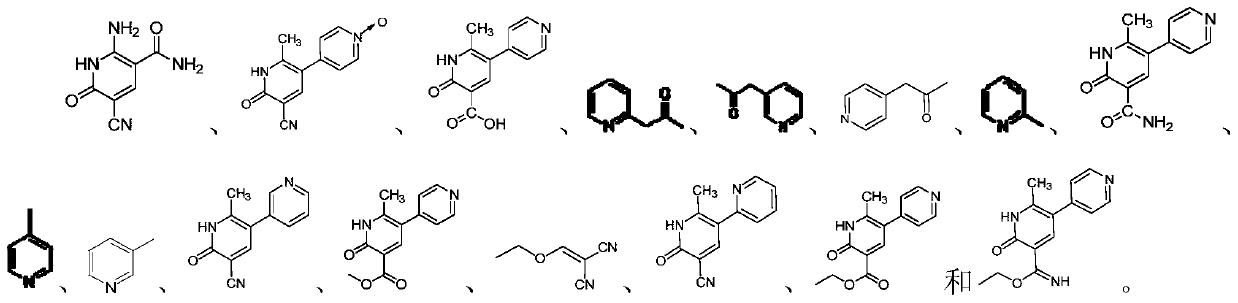
Preparation method of milrinone impurities
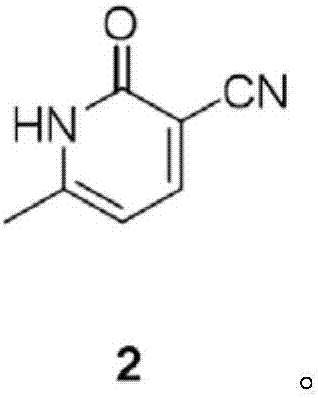
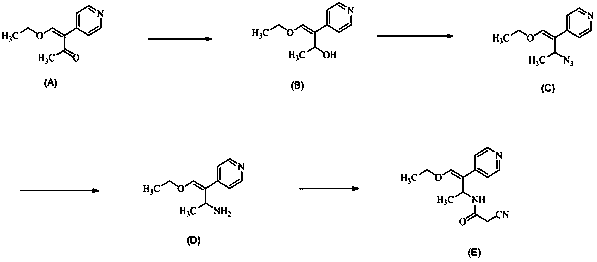
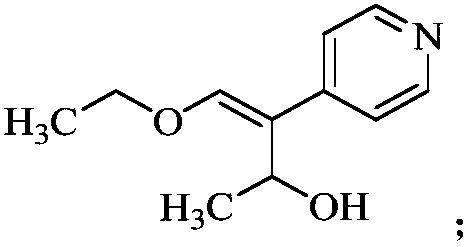
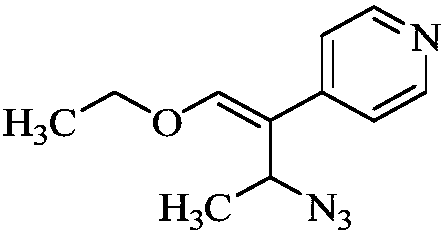
The invention discloses a preparation method of milrinone impurities, and belongs to the field of pharmaceutical synthesis. The preparation method disclosed by the invention has the advantages of reasonable design of reaction routes and high operability. The method comprises the following steps: by using (Z)-4-ethoxyl-3-(4-pyridyl)-3-ene-2-butanone as a raw material, performing four-step reactionsynthesis including reduction, azidation, azide reduction and condensation between the raw material and cyanoacetic acid so as to synthesize a target compound. According to the method disclosed by the invention, the design of the whole route is reasonable, post-treatment is simple, and the raw material is low-cost and easy to obtain; the purity of the target product prepared by the method disclosed by the invention can reach 99.0 percent or above, and can be applied to pharmacokinetics and clinical research; moreover, a testing sample is provided for clinical research on milrinone, and the target product has an important application value.
Owner:TLC NANJING PHARMA RANDD CO LTD
Injecta containing milrinone and preparation method thereof
ActiveCN102727431AGood effectProlong survival timeOrganic active ingredientsPharmaceutical delivery mechanismMilrinoneAnaerobic respiration
The invention discloses injecta containing milrinone and a preparation method of the injecta. The injecta contains the components of 1g / L of milrinone, 0.5-2g / L of amiodarone hydrochloride, 0.8-1.0% (w / v) of sodium chloride and 4-10% (v / v) of ethanol. The dosage of a pharmaceutically acceptable acidic material is that pH of the injecta is 3.0-5.0, and the balance is injection water. According to the injecta disclosed by the invention, the symptoms of hemorrhagic shock can be effectively improved; generation of anaerobic respiration lactic acid is restrained under an anaerobic condition; the injecta has a good function on protecting liver and kidney, and the survival time of a sufferer is obviously prolonged.
Owner:NANJING CHIA TAI TIANQING PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com