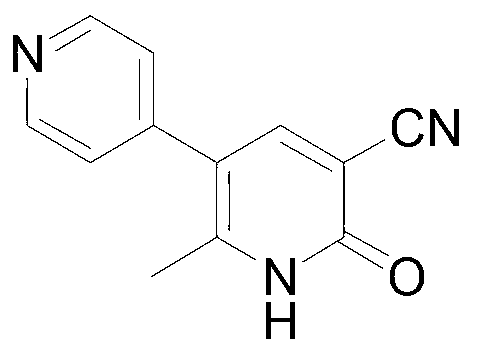
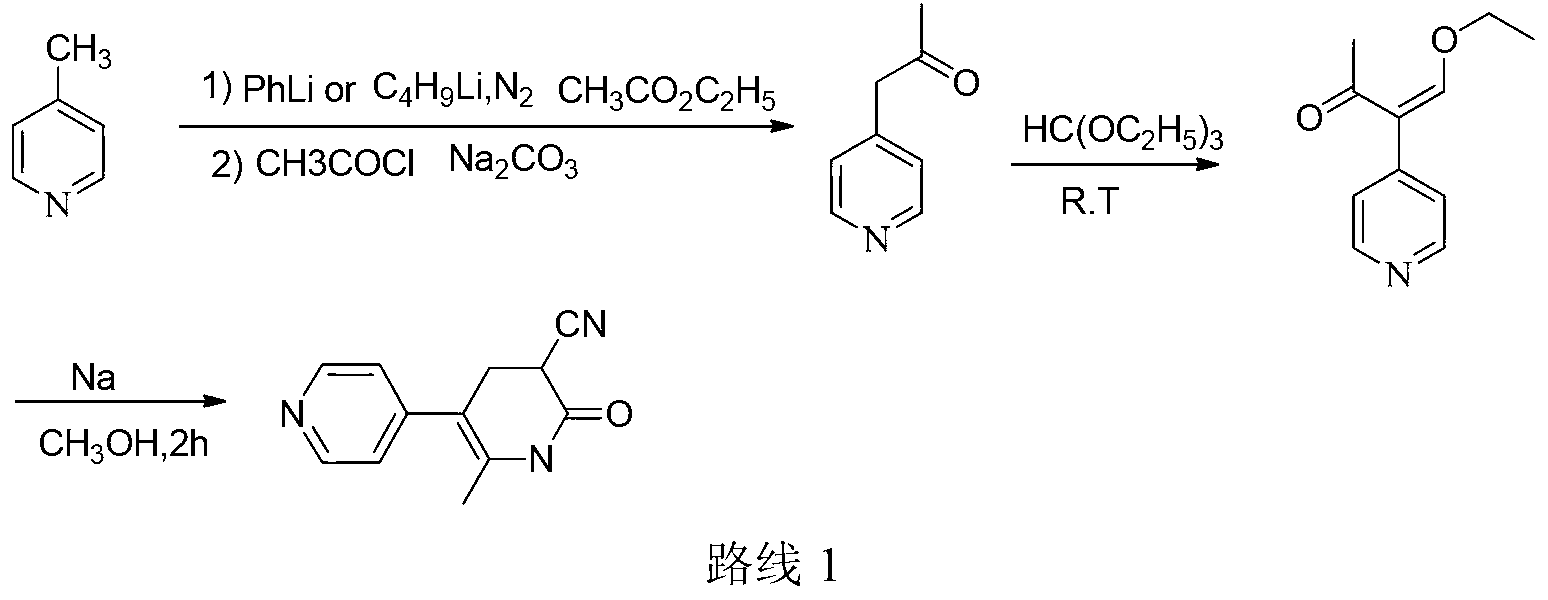
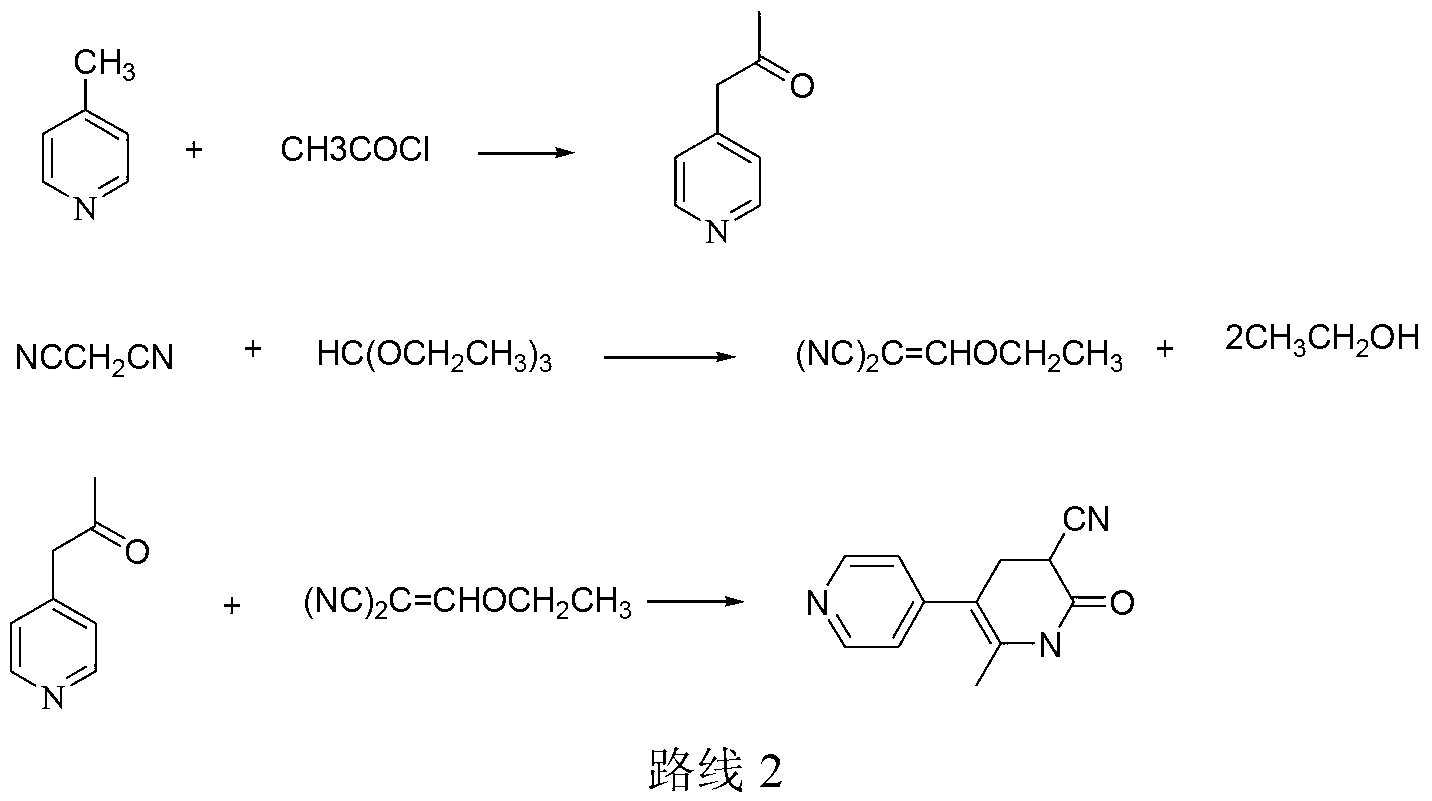
Method for synthesising milrinone
A technology for synthesizing rice and compounds, which is applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, long reaction time, high price, etc., and achieve the effects of reducing the number of times of extraction, decompression and concentration, facilitating industrial production, and saving labor costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] In a 100mL three-necked flask, add 7.95mL (81mmol) of 4-picoline, 30mL of dichloromethane and 20mg of anhydrous aluminum chloride, cool in an ice bath to 0°C, and dropwise add 11.7mL (165mmol) of acetyl chloride. Control the internal temperature below 10°C, raise the temperature to 25°C after the dropwise addition, and react for 2.5 hours. Under cooling in an ice bath, add 0.5 mol / L sodium hydroxide aqueous solution dropwise to the system to adjust the pH to 7-8, then add 19.5 mL saturated sodium bisulfite solution, and stir at room temperature for 2.5 hr. The combined organic layers were extracted with dichloromethane (50 mL×2), and dried over anhydrous sodium sulfate. After the solvent was recovered, 3.84 g of a fraction at 65-67°C / 2.7kPa was collected by vacuum distillation, which was unreacted 4-methylpyridine. The aqueous layer was adjusted to pH 13.25 with 6.25mol·L-1 sodium hydroxide solution, and reacted at room temperature for 2 hours. Add 24 mL of water, ext...
Embodiment 2
[0032] In a 1000mL three-necked flask, add 63.6mL (0.648mol) of 4-methylpyridine and 240mL of n-hexane, and add 66.9mL (0.972mol) of acetyl chloride dropwise under cooling in an ice bath, and control the internal temperature below 10°C during the dropwise addition , After the dropwise addition, the temperature was raised to 40° C. for reflux reaction, and the reaction was carried out for 3 hours. Add 6 mol·L dropwise to the system under cooling in an ice bath -1Adjust the pH to 7-8 with aqueous sodium hydroxide solution, then add 156 mL of saturated sodium bisulfite solution, and stir at room temperature for 2.5 hr. The combined organic layers were extracted with n-hexane (150 mL×2), and dried over anhydrous sodium sulfate. After recovering the solvent, 31.6 g of fractions at 65-67° C. / 2.7 kPa were collected by vacuum distillation, which was unreacted 4-picoline. The aqueous layer was adjusted to pH 13.08 with 6.25 mol·L-1 sodium hydroxide solution, and reacted at room tempe...
Embodiment 3
[0035] In a 2000mL three-necked flask, add 200mL (2.038mol) of 4-picoline, 800mL of cyclohexane and 1mL of copper trifluoromethanesulfonate, and add 361.3mL (5.092mol) of acetyl chloride dropwise under cooling in an ice bath. Control the internal temperature below 10°C, and raise the temperature to 30°C for 3.5 hours after the dropwise addition. Under cooling in an ice bath, 4 mol·L-1 sodium hydroxide aqueous solution was added dropwise to the system to adjust the pH to 7-8, then 491 mL of saturated sodium bisulfite solution was added, and stirred at room temperature for 2.5 hr. The combined organic layers were extracted with cyclohexane (400 mL×2), and dried over anhydrous sodium sulfate. After recovering the solvent, 93.95 g of fractions at 65-67°C / 2.7kPa were collected by vacuum distillation, which was unreacted 4-picoline. The aqueous layer was adjusted to pH 13.18 with 6.25 mol·L-1 sodium hydroxide solution, and reacted at room temperature for 2 hours. Add 600 mL of wat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com