Method for analyzing milrinone related substances by high performance liquid chromatography
A technology for high performance liquid chromatography and related substances, which is applied in the field of analyzing milrinone related substances, can solve the problems such as the need for further improvement of quality control, the limited detection ability of impurities, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Analytical method is established
[0036] Instrument: Agilent 1100 / 1260 HPLC
[0037] Column: Agilent ZORBAX SB-C 8 (250×4.6mm, 5μm)
[0038] Mobile phase A: Dipotassium hydrogen phosphate solution at pH 7.5 (take 2.7g of dipotassium hydrogen phosphate, add 800ml of water to dissolve, add 2.4ml of triethylamine, adjust the pH value to 7.5 with phosphoric acid)
[0039] Mobile phase B: water-acetonitrile (50:50)
[0040] The flow rate is 1ml / min; the column temperature is 30°C; the detection wavelength is 260nm; the injection volume is 20μl.
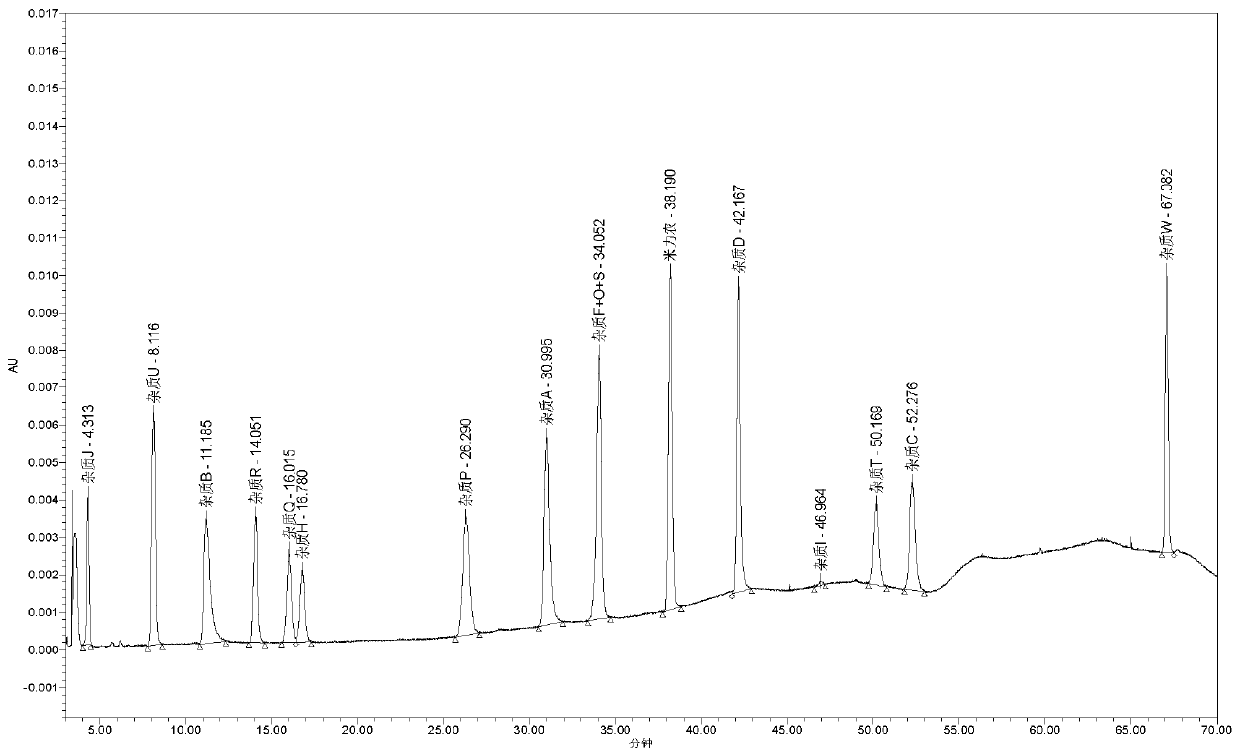
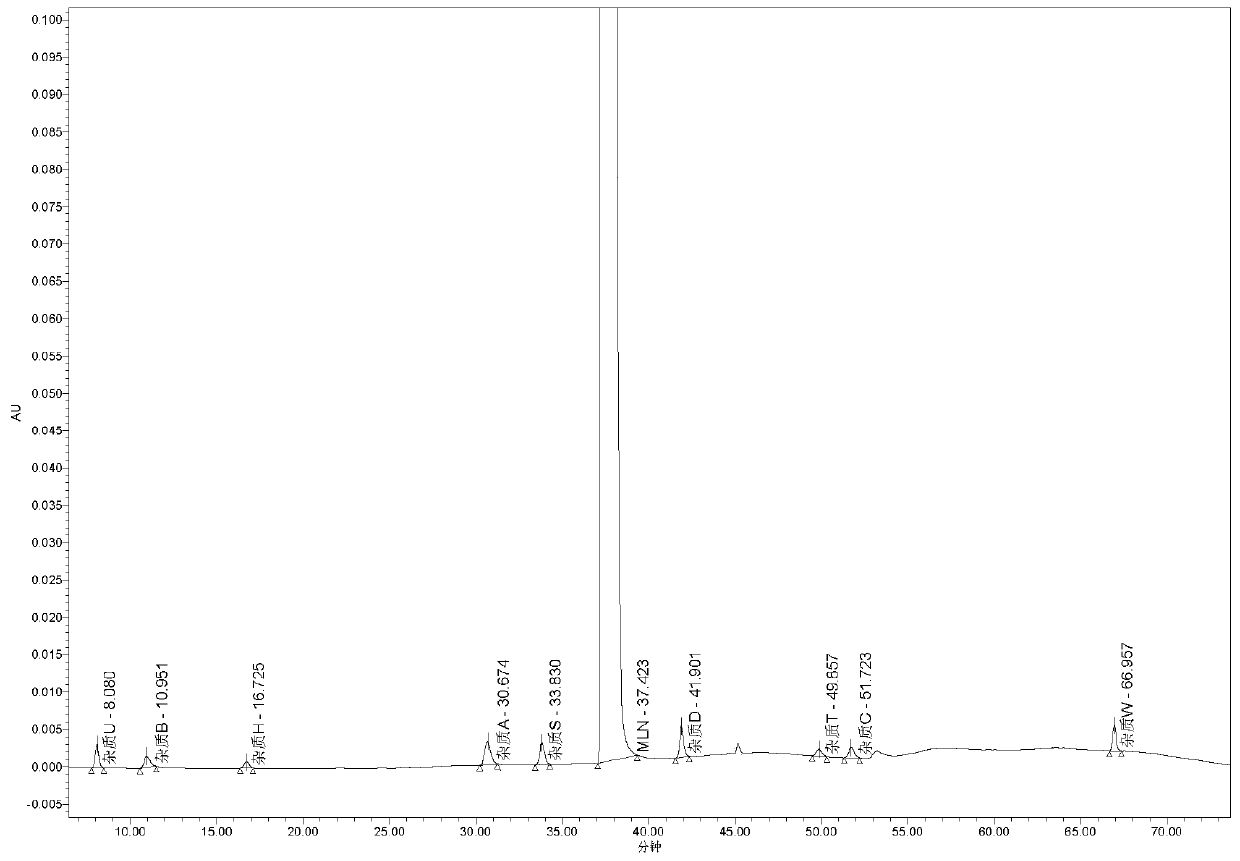
[0041] The elution procedure is shown in Table 2, and the obtained spectrum can be found in figure 1 , Realized the characterization of impurities in 13, wherein 4 kinds of impurities are non-critical impurities of this product, which are not included in the method of this embodiment, but this method can still effectively detect the impurities that are not reflected.
[0042] Table 2
[0043]
[0044] System s...
Embodiment 2
[0052] Embodiment 2 durability test
[0053] Fine-tune the temperature (±5°C), flow rate (±20%), detection wavelength (±2nm), mobile phase ratio, and initial gradient ratio to evaluate the tolerance of the measurement results without being affected by small changes in the parameters of the measurement conditions. The test results are shown in Table 4.
[0054] Table 4
[0055]
[0056]
[0057] It can be seen from Table 4 that the detection method of milrinone-related substances can meet the system applicability requirements by adjusting the column temperature, flow rate, initial mobile phase ratio, and detection wavelength, indicating that the method has good durability.
Embodiment 3
[0058] Embodiment 3 detection limit
[0059] For known impurities: prepare a solution with a concentration of 0.025% of the test substance (at least half of the impurity limit concentration), if the signal-to-noise ratio S / N is not less than 10, then use the concentration of 0.025% of the test substance as the limit of quantification; For a solution with a concentration of 0.01% of the test substance, if the signal-to-noise ratio S / N is not less than 2, then the concentration of 0.01% of the test substance is the detection limit concentration;
[0060] For unknown impurities: replace the quantification limit concentration and detection limit concentration of a single unknown impurity with principal components.
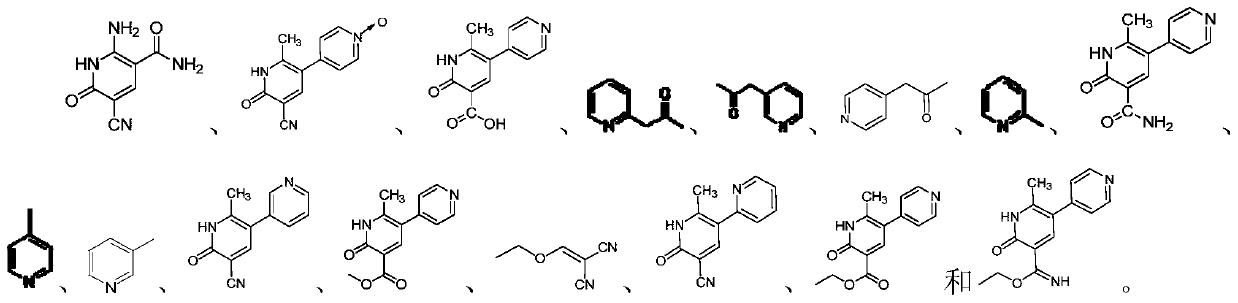
[0061] After testing, the detection limit concentration of impurity U, impurity B, impurity H, impurity A, impurity S, milrinone, impurity D, impurity T, impurity C and impurity W were 0.2022 μg / ml, 0.2010 μg / ml, 0.2237 μg / ml, 0.1984 μg / ml, 0.1981 μg / ml, 0.2037 μg / ml,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com