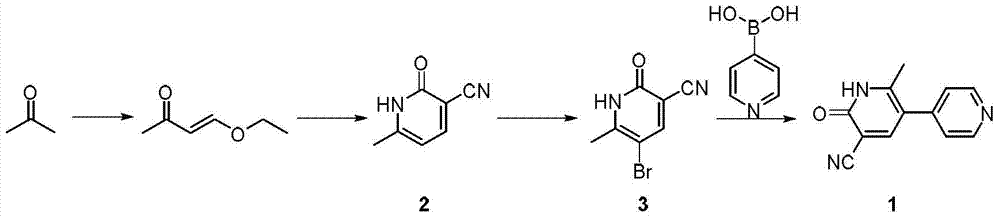
A kind of method of synthesizing milrinone
A technology for synthesizing rice and compounds, applied in the field of medicine, can solve problems such as serious pollution, poor operating environment, and non-synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
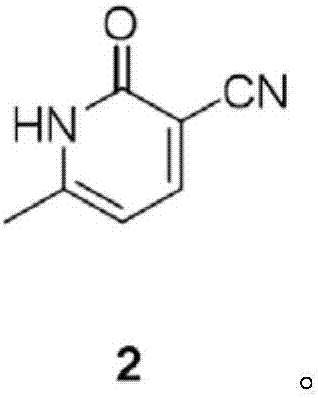
[0048] Synthesis of Compound 2
[0049] Add 46.5g of sodium methoxide in 950ml of tetrahydrofuran (THF) solution to the reaction vessel, cool in an ice bath to an internal temperature of 0-5°C, add a solution of 46.5g of acetone and 59.6g of ethyl formate dropwise within 1 hour, and remove the ice after the addition is complete. bath, slowly rose to room temperature within 1h, and concentrated under reduced pressure (not exceeding 50°C). Transfer the concentrate to a reaction vessel, add 400ml aqueous solution of 67g cyanoacetamide and piperidine acetic acid (condensation reaction catalyst), reflux for 2h, cool to room temperature, adjust pH=5 with acetic acid, stand at room temperature overnight, and cool in an ice bath After 45 minutes, it was filtered with suction, washed with ice water three times, and dried in vacuum at 80° C. overnight to obtain 65.5 g of compound 2 as a yellow solid (yield 61.0%).
[0050] 1 H NMR (DMSO-d 6 )δ 12.50 (br s, 1H), 8.03 (d, 1H), 6.21 (d,...
Embodiment 2
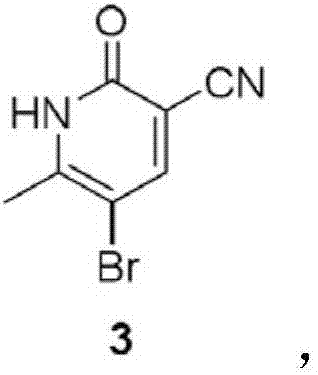
[0052] Add 26.2g of compound 2 and 36.5g of NBS in 250ml of dichloroethane solution to the reaction vessel, heat to reflux for 18h, cool to room temperature, filter, the solid is slurried with 500ml of water for 2h, filter, wash with water, and dry in vacuo to obtain 38.4g of compound as a white solid 3 (92.3% yield).
[0053] 1H NMR (DMSO-d 6 )δ12.92(br s,1H), 8.30(s,1H), 2.35(s,3H).
Embodiment 3
[0055] Add 21.3g of compound 3, 12.9g of 4-pyridineboronic acid, 50ml of toluene, 50ml of 2M aqueous sodium hydroxide solution, 2.3g of tetrakis(triphenylphosphine)palladium to the reaction vessel, reflux for 18h under nitrogen atmosphere, cool to room temperature, and add 10ml 50% sodium hydroxide aqueous solution was stirred for 15 minutes, and the water layer was separated. The pH of the water layer was adjusted to about 6 with acetic acid, and a solid was precipitated. The yield was 15.3 g of milrinone (yield 72.5%) as a white solid.
[0056] 1 H NMR (DMSO-d 6 )δ 12.86 (br s, 1H), 8.62 (d, 2H), 8.20 (s, 1H), 7.40 (d, 2H), 2.31 (d, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com