Preparation method for high-purity milrinone
A milrinone, high-purity technology, applied in the field of medicine, can solve the problems of strict requirements on water for phenyllithium, relatively high reaction conditions, and the influence of active ingredients in preparations, and achieves improved production environment, high product purity, and solubility. better results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
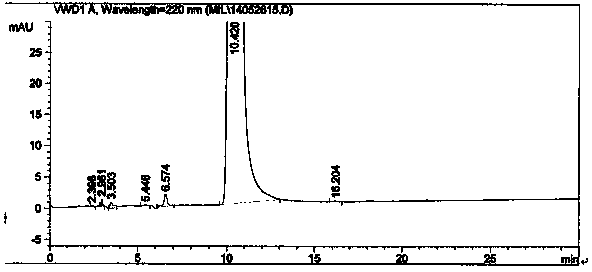
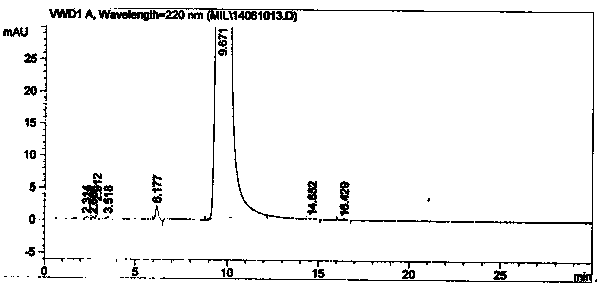
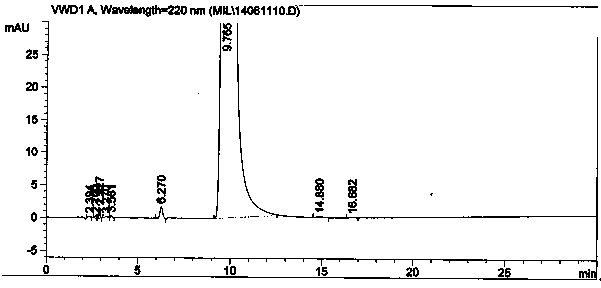
Image
Examples
example 1
[0050] In a 1000mL three-necked flask, add 93.0g (1.0mol) of 4-methylpyridine and 500mL of chloroform, place in an ice water bath and control the temperature below 50℃, add 80.0g (1.02mol) of acetyl chloride dropwise, after the addition is complete The temperature was raised to 55°C for 2.5hr. After the reaction is completed, a saturated aqueous sodium carbonate solution is added dropwise to the system under ice cooling to adjust the pH to 5-7, and then 30.0g 30wt% sodium hydroxide solution (0.23mol sodium hydroxide) is added, and the reaction is stirred at 30-50°C for 2.5hr. After the reaction is completed, separate the layers, remove the water layer, dry with anhydrous sodium sulfate, recover the solvent, and collect the 100-105°C / 217kPa fraction, namely 1-(4-pyridyl)-2-acetone, by distillation under reduced pressure, the fraction 97.2g, HPLC: 98.4%, the yield is 72.08%.
[0051] Add 60.0 (0.44mol) g of 1-(4-pyridyl)-2-acetone into a 500mL round-bottomed flask, mix 40.5g (...
example 2
[0054] In a 5000mL three-necked flask, add 465.0g (5.0mol) of 4-methylpyridine and 3000mL of chloroform, place it in an ice water bath to control the temperature below 50℃ and add 588.8g (7.5mol) of acetyl chloride dropwise, after the addition is complete The temperature was raised to 55°C for 3.5hr. After the reaction is completed, a saturated aqueous sodium carbonate solution is added dropwise to the system under ice cooling to adjust the pH to 5-7, and then 160.0g 30% sodium hydroxide solution (1.2mol sodium hydroxide) is added, and the reaction is stirred at 30-50°C for 2.5hr. After the reaction is complete, separate the layers, remove the water layer, dry with anhydrous sodium sulfate, recover the solvent and distill under reduced pressure to collect 100-105°C / 217kPa fraction, namely 1-(4-pyridyl)-2-acetone, fraction 498.2g, HPLC: 98.2%, yield 73.8%.
[0055] Add 280.0g (2.07mol) of 1-(4-pyridyl)-2-acetone into a 3000mL round-bottomed flask, mix 572.0g (6.21mol) of triethyl...
example 3
[0058] In a 10000mL three-necked flask, add 930.0 g (10mol) of 4-methylpyridine and 6500mL of chloroform, place in an ice water bath and control the temperature below 50℃. Add 981.0 g (12.5mol) of acetyl chloride dropwise. React at 55°C for 3.0hr. After the reaction is completed, add saturated sodium carbonate aqueous solution to the system under ice-bath cooling to adjust the pH to 5-7, then add 315.0g 30% sodium hydroxide solution (2.36mol), stir and react at 30-50℃ for 3.0hr. After the reaction is complete, separate the layers, remove the water layer, dry with anhydrous sodium sulfate, recover the solvent and distill under reduced pressure to collect the 100-105°C / 217kPa fraction, namely 1-(4-pyridyl)-2-acetone, fraction 996.0g, HPLC: 98.2%, yield 73.8%.
[0059] Add 600.0g (4.44mol) of 1-(4-pyridyl)-2-acetone into a 5000mL round-bottomed flask, mix 817.0g (8.87mol) of triethyl orthoformate, 1133.0g (11.10mol) of acetic anhydride and 1201.0 (20.00 mol) glacial acetic acid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com