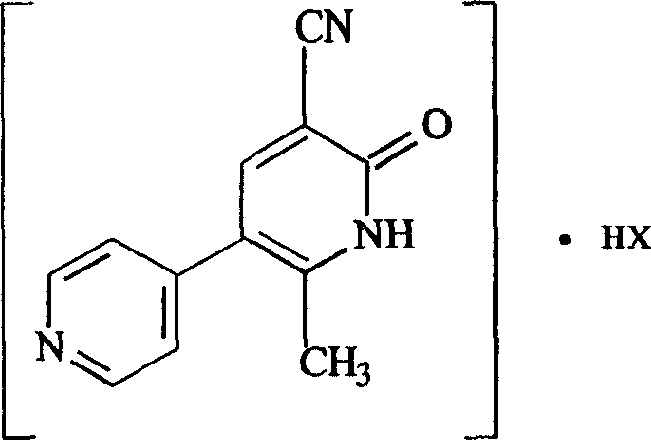
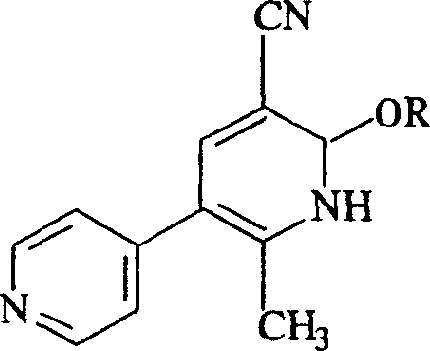
Milrinone salt preparation method and its uses
A technology of milrinone and hydrochloric acid, applied in the fields of chemical industry and chemical medicine, can solve the problems of inconvenient transportation, difficult storage, poor stability, etc., and achieve the effect of solving the problems of solubility and stability, and being convenient for storage and transportation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
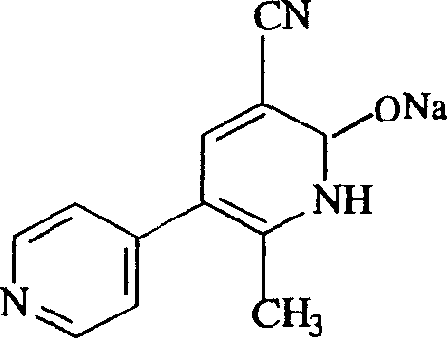
[0031] Embodiment 1: the preparation of milrinone sodium salt
[0032] Put 10g of milrinone in a 500ml beaker, add dropwise 0.1N sodium hydroxide aqueous solution and stir to dissolve it until the pH value is 7-8, add acetone 5 times the volume of the solution, cool, and precipitate a white precipitate, filter, filter The cake was washed twice with acetone, dried in air, and then dried at 105° C. for 2 hours to obtain 10.16 g of milrinone sodium salt with a yield of 92%.
[0033] Elemental Analysis: C 12 h 8 N 3 ONa, calculated C: 61.74% H: 3.43% N: 18.01% Na: 9.86%, experimental value C: 61.70% H: 3.38% N: 17.94% Na: 9.83%;
[0034] Infrared spectrum (KBr, cm -1 ): 3636 3264 3057 2214 1594 1550 1522 14821400 1367 1259 827 807 789 601
[0035] UV absorption spectrum: determination of maximum lambda in aqueous solution max 333nm, 269nm.
Embodiment 2
[0036] Embodiment 2. Preparation of milrinone potassium salt
[0037] Put 10g of milrinone in a 500ml beaker, add 0.1N potassium hydroxide solution dropwise and stir to dissolve it until the pH value of the solution is 7-8, add acetone 5 times the volume of the solution, cool, and precipitate a white precipitate, filter, The filter cake was washed twice with acetone, dried in air, and then dried at 105° C. for 2 hours to obtain 5.0 g of milrinone potassium salt, and 4.5 g was recovered from the mother liquor, with a yield of 80.5%.
[0038] Elemental Analysis: C 12 h 8 N 3 OK, calculated value C: 57.78% H: 3.21% N: 16.85% K: 15.64%, experimental value C: 57.63% H: 3.23% N: 16.80% K: 15.60%
[0039] Infrared spectrum (KBr, cm -1 ): 3335 3032 2213 1596 1549 1525 1480 13981367 1270 1226 830 790 597
[0040] UV absorption spectrum: determination of maximum lambda in aqueous solution max 334nm, 266nm.
Embodiment 3
[0041] Embodiment 3. The preparation of milrinone hydrochloride
[0042] Put 10g of milrinone in a 500ml beaker, add dropwise 0.1N hydrochloric acid solution and stir to dissolve it, so that the pH of the solution is 4-4.5, add acetone 5 times the volume of the solution, cool, and precipitate a white precipitate, filter, filter cake Wash twice with acetone, dry in air, and then dry at 105° C. for 2 hours to obtain 12 g of milrinone hydrochloride with a yield of 93%.
[0043] Elemental analysis; C 12 h 9 N 3 O HCl, calculated value C: 58.11% H: 4.07% N: 16.56% Cl: 14.31%, experimental value C: 58.11% H: 4.07% N: 16.56% Cl: 13.98%
[0044] Infrared spectrum (KBr, cm -1 ): 3223 3026 2987 2784 2627 2235 1873 1654 1626 1570 1511 1478 1371 1336 1182 1031 830 687 583
[0045] UV absorption spectrum: determination of maximum lambda in aqueous solution max 331nm, 265nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com