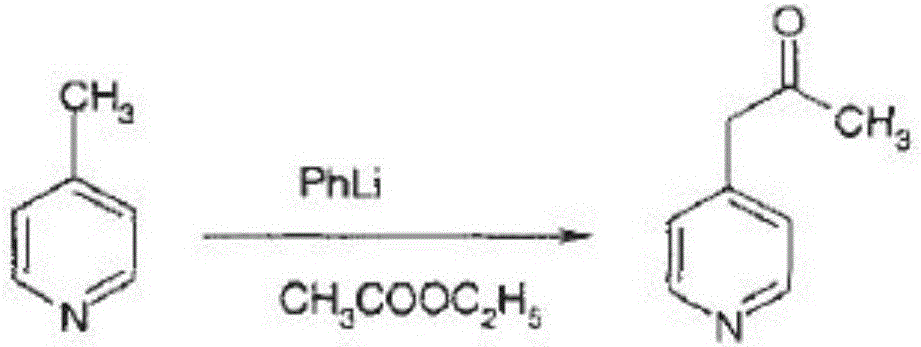Method for preparing milrinone
A milrinone and pyridyl-based technology, which is applied in the field of preparation of milrinone, can solve the problems of safety hazards, harsh conditions, and instability in personal health, and achieve easy industrial promotion, mild reaction conditions, and no safety hazards Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The invention provides a kind of preparation method of milrinone, comprises the following steps:
[0046] (1) Preparation of 1-(4-pyridyl)-acetone
[0047] a. First mix 4-picoline and maleic anhydride in the solvent, then add the catalyst, keep the temperature at 30-45°C, keep stirring, and slowly add propanol to react for 30-60 minutes;
[0048] b. Evaporate the reactant obtained in step a, then extract with acetone, and finally dry and filter with anhydrous copper sulfate to obtain 1-(4-pyridyl)-acetone;
[0049] (2) Preparation of 1-ethoxy-2-(4-pyridyl) vinyl methyl ketone
[0050] a. Add triethyl orthoformate, maleic anhydride and maleic acid to the 1-(4-pyridyl)-acetone prepared in step (1), maintain the temperature at 20-45° C., and react for 1-3 hours;
[0051] b. Evaporate the reactant obtained in step a to remove maleic anhydride and maleic acid to obtain 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone;
[0052] (3) Preparation of Milrinone
[0053] a. Add ethano...
Embodiment 1
[0081] 1, the preparation of 1-(4-pyridyl)-acetone
[0082] In a three-necked flask, add 1mol 4-picoline, 2mol maleic anhydride and 500ml n-hexane and mix well, add 3ml sulfuric acid dropwise, keep the temperature at 35-40°C in an ice-water bath, keep stirring, and slowly add 500ml propane Alcohol, react for 30 minutes; when the temperature is 55-65°C, evaporate the above reactant for 60 minutes to remove excess propanol, then add 300ml acetone for extraction, extract three times, evaporate to remove excess acetone, and finally use anhydrous copper sulfate Dry and filter to obtain 1-(4-pyridyl)-acetone.
[0083] 2. Preparation of 1-ethoxy-2-(4-pyridyl) vinyl methyl ketone
[0084] Add 1 mol of 1-(4-pyridyl)-acetone, 2 mol of triethyl orthoformate, 2.5 mol of maleic anhydride and 3 mol of maleic acid into a three-necked flask, stir, and maintain the temperature at 30-40°C in an ice-water bath. Reaction for 1.5h; at a temperature of 50-60°C, evaporate the above reactant for 1....
Embodiment 2
[0088] 1, the preparation of 1-(4-pyridyl)-acetone
[0089] In a three-necked flask, add 1mol 4-picoline, 3mol maleic anhydride and 500ml n-hexane and mix well, add 5ml sulfuric acid dropwise, keep the temperature at 35-40°C in an ice-water bath, keep stirring, and slowly add 500ml propane Alcohol, react for 40 minutes; when the temperature is 55-65°C, evaporate the above reactant for 50 minutes to remove excess propanol, then add 300ml acetone for extraction, extract three times, evaporate to remove excess acetone, and finally use anhydrous copper sulfate Dry and filter to obtain 1-(4-pyridyl)-acetone.
[0090] 2. Preparation of 1-ethoxy-2-(4-pyridyl) vinyl methyl ketone
[0091] Add 1 mol of 1-(4-pyridyl)-acetone, 2.2 mol of triethyl orthoformate, 2.5 mol of maleic anhydride and 3 mol of maleic acid into a three-necked flask, stir, and maintain the temperature at 30-40°C in an ice-water bath , reacted for 2h; when the temperature was 50-60°C, evaporate the above reactant f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com