Preparation method of milrinone impurities
A technology of milrinone and impurities, applied in the field of drug synthesis, to achieve the effects of controllable reaction, reasonable route design and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
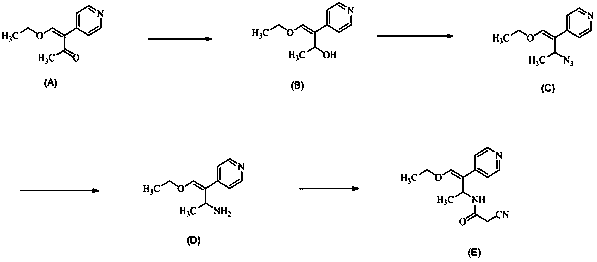
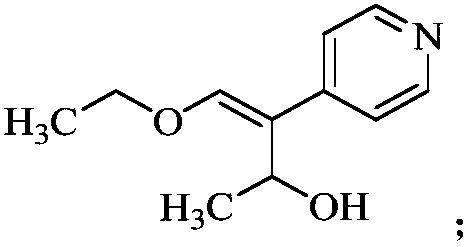
[0019] (1) Dissolve 24.4g of (Z)-4-ethoxy-3-(4-pyridyl)-3-ene-2-butanone in 360mL of methanol, and add 4.8g of sodium borohydride in batches , after stirring and reacting at room temperature for 3 hours, thin-layer chromatography showed that the reaction was complete, the organic phase was washed with water, dried over anhydrous sodium sulfate, and concentrated to obtain 21g of yellow-brown oily compound B, with a yield of 85.17%;
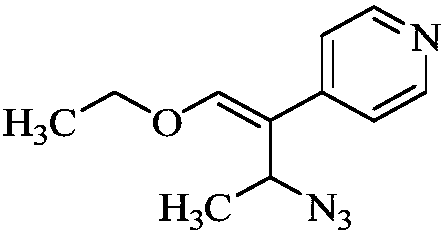
[0020] (2) Dissolve 21g of compound B in 210mL of toluene, add diphenylphosphoryl azide, stir and react at 30°C for 4 hours, thin-layer chromatography shows that the reaction is complete, add 200mL of water to the mixture, and the product is dichloromethane Extraction, the organic phase was dried with anhydrous sodium sulfate to obtain 22g of compound C, yield 92.72%;
[0021] (3) Dissolve 16g of compound C in 320mL of methanol and 64mL of water, add 40g of triphenylphosphine, and react at 25°C for 2 hours. Thin layer chromatography shows that the ...
Embodiment 2
[0024] (1) Dissolve 24.4g of (Z)-4-ethoxy-3-(4-pyridyl)-3-ene-2-butanone in 360mL of methanol, and add 6.8g of potassium borohydride in batches , after stirring and reacting at room temperature for 3 hours, thin-layer chromatography showed that the reaction was complete, the organic phase was washed with water, dried over anhydrous sodium sulfate, and concentrated to obtain 21g of yellow-brown oily compound B, with a yield of 85.17%;
[0025] (2) Dissolve 21g of Compound B in 210mL of toluene, add sodium azide, stir and react at 30°C for 4 hours, thin-layer chromatography shows that the reaction is complete, add 200mL of water to the mixture, and extract the product with dichloromethane. The organic phase was dried with anhydrous sodium sulfate to obtain 22 g of compound C, with a yield of 92.72%;
[0026] (3) Dissolve 16g of compound C in 320mL of ethanol and 64mL of water, add 18g of triethylphosphine, and react at 25°C for 2 hours. Thin layer chromatography shows that the r...
Embodiment 3
[0029] (1) Dissolve 24.4g of (Z)-4-ethoxy-3-(4-pyridyl)-3-ene-2-butanone in 360mL of methanol, and add 6.8g of potassium borohydride in batches After stirring and reacting at room temperature for 4 hours, thin-layer chromatography showed that the reaction was complete. The organic phase was washed with water, dried over anhydrous sodium sulfate, and concentrated to obtain 21 g of yellow-brown oily compound B, with a yield of 85.17%;
[0030] (2) Dissolve 21g of Compound B in 210mL of toluene, add sodium azide, stir and react at 30°C for 5 hours, thin layer chromatography shows that the reaction is complete, add 200mL of water to the mixture, and extract the product with dichloromethane. The organic phase was dried with anhydrous sodium sulfate to obtain 22 g of compound C, with a yield of 92.72%;
[0031] (3) Dissolve 16g of compound C in 320mL of ethanol and 64mL of water, add 11.6g of trimethylphosphine, and react at 25°C for 2 hours. Thin layer chromatography shows that the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com