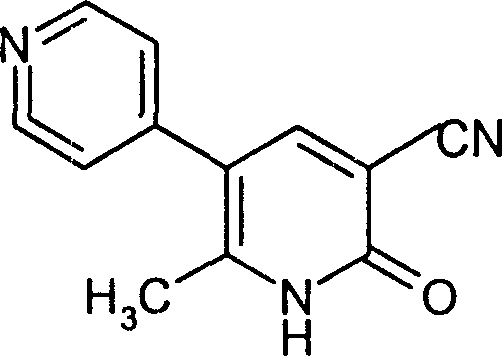
Milrinone lyophilized powder for injection and preparation method thereof
A technology for freeze-dried powder and injection, applied in the field of milrinone freeze-dried preparations and its preparation, can solve the problem that the stability of water injection is not as stable as that of freeze-dried powder injection, and achieve fine frozen crystals, good stability, and fast dissolution Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0030] Example 1. (1000 pieces)
[0031] Milrinone 5g
[0032] Mannitol 50g
[0033] Adjust pH to 2.5 with lactic acid or sodium hydroxide
[0034] Add water for injection to 1000ml
[0035] Dissolve milrinone in 750ml of water for injection, add lactic acid or sodium hydroxide to adjust the pH to 2.5, and sonicate for 20 minutes until it is completely dissolved. Then add mannitol and stir for 20 minutes to fully dissolve. Add water for injection to full volume. Then filter with a sterilizing filter, fill in a control antibiotic glass bottle, stopper the plate and put it in a freeze dryer. Pre-freezing: Put the subpackaged medicines on the inner partition of the freeze-drying box for pre-freezing, and keep the product temperature for 2 hours after the temperature drops to -40°C. Sublimation stage: When the vacuum degree in the drying box reaches below 13.33Pa (0.1mmg column), turn off the freezer, heat slowly through the partition, and properly heat the partition to -12°...
Embodiment 3~4
[0036] Embodiment 3~4 (1000)
[0037] Milrinone 20g
[0038] Glucose 600g
[0039] Adjust pH to 3.0 with lactic acid or sodium hydroxide
[0040] Add water for injection to 6000ml
[0041] Dissolve the prescribed amount of milrinone in 4500ml of water for injection, add an appropriate amount of lactic acid or sodium hydroxide to adjust the pH to 3.0, and sonicate for 20 minutes until it is completely dissolved. Then add glucose, stir for 20min to fully dissolve. Add water for injection to full volume. Then filter with a sterilizing filter, fill in a control antibiotic glass bottle, stopper the plate and put it in a freeze dryer. Pre-freezing: Put the subpackaged medicines on the inner partition of the freeze-drying box for pre-freezing, and keep the product temperature for 3 hours after dropping to -40°C. Sublimation stage: When the vacuum degree in the drying box reaches below 13.33Pa (0.1mmg column), turn off the freezer, heat slowly through the partition...
Embodiment 5~6
[0042] Embodiment 5~6 (1000)
[0043] Milrinone 1g
[0044] Mannitol 40g
[0045] Adjust pH to 2.7 with lactic acid or sodium hydroxide
[0046] Add water for injection to 400ml
[0047] Dissolve the prescribed amount of milrinone in 3 / 4 volume of water for injection, add appropriate amount of lactic acid or sodium hydroxide to adjust the pH value to 2.7, and sonicate for 20 minutes until completely dissolved. Then add mannitol and stir for 20 minutes to fully dissolve. Add water for injection to full volume. Then filter with a sterilizing filter, fill in a control antibiotic glass bottle, stopper the plate and put it in a freeze dryer. Pre-freezing: Put the subpackaged medicines on the inner partition of the freeze-drying box for pre-freezing, and keep the product temperature for 2 hours after the temperature drops to -40. Sublimation stage: When the vacuum degree in the drying box reaches below 13.33Pa (0.1mmg column), turn off the freezer, heat slow...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com