A kind of preparation method of milrinone
A technology of milrinone and monohydric alcohol, which is applied in the field of medicine, can solve the problems of increasing impurities, reducing purity, and high cost, and achieves the effects of improving product purity, saving production costs, and simplifying process steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
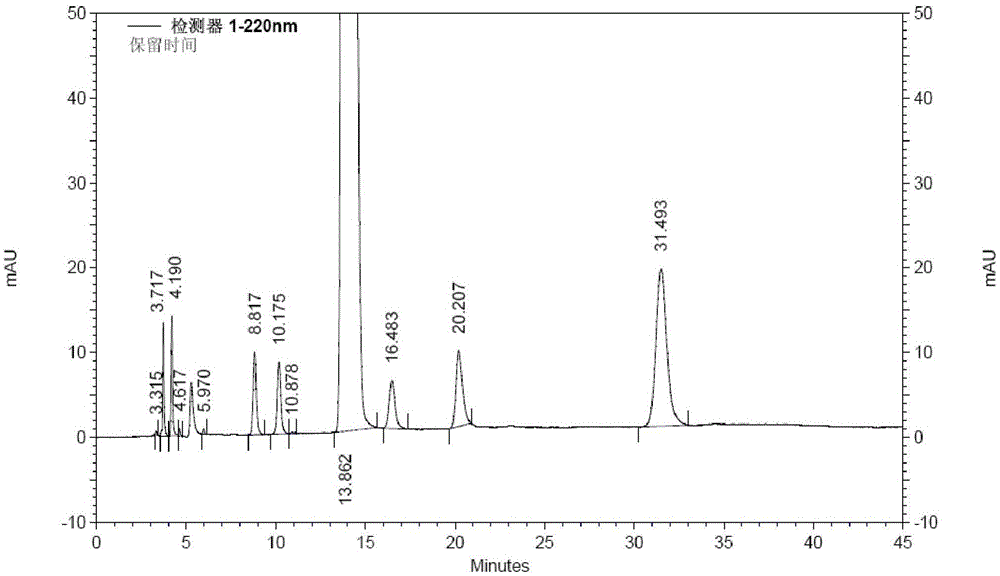
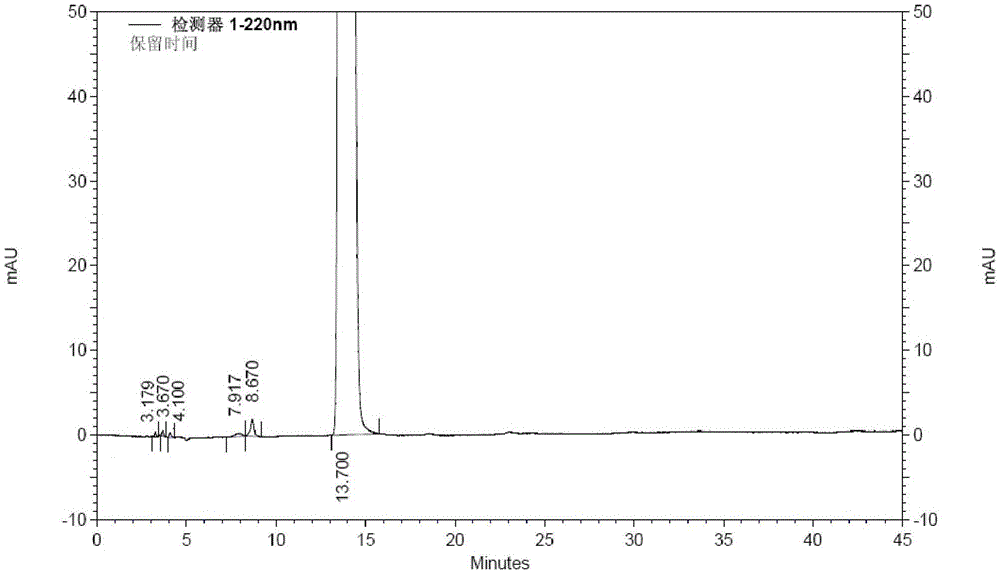
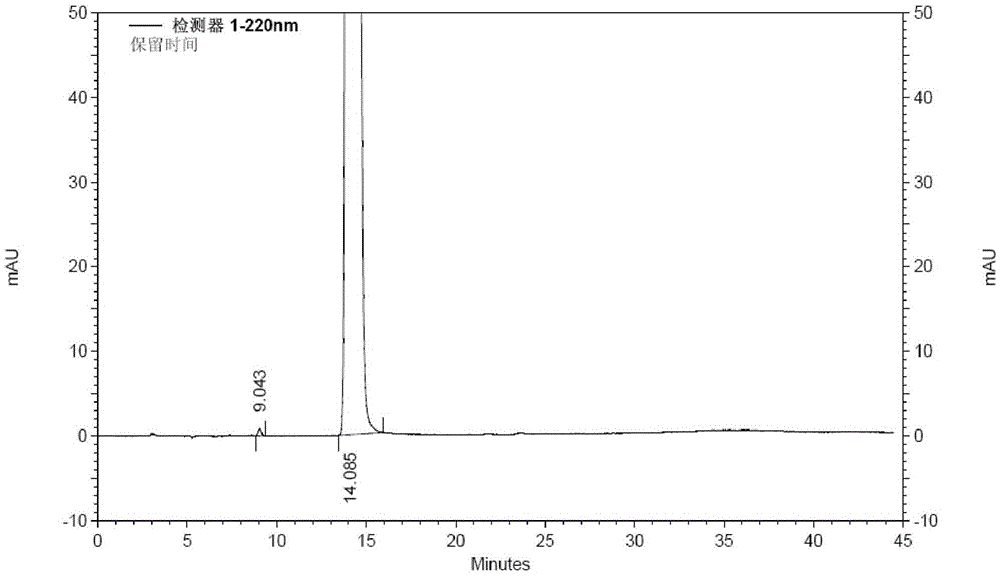
[0039] Take 200g (1.05mol) of 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone, add 2L of 80vt% ethanol to dissolve, add 97g (1.15mol) of cyanoacetamide, stir to dissolve, add dropwise hydrogen oxidation Sodium 80vt% ethanol solution 630ml (sodium hydroxide content 126g), temperature control below 0°C; after dripping, stir and react at -5~0°C for 16h, add 1.3L of water to the reaction solution, add 50g of activated carbon, stir at room temperature for 20min, filter, Adjust the pH of the filtrate to 7 with hydrochloric acid solution, filter, wash the filter cake with water until the filtrate is colorless, add 200 g of wet dexmilrinone, add 4 L of 50vt% ethanol to reflux to dissolve, filter, stir and crystallize the filtrate at -5~0°C for 6 hours, filter, After drying at 60-70°C for 6 hours, 183 g of white crystalline milrinone was obtained, with a yield of 82.8% and a purity of 99.97% (HPLC method).
Embodiment 2
[0041] Take 2 kg (10.5 mol) of 1-ethoxy-2-(4-pyridyl) vinyl methyl ketone, add 22 L of 70vt% ethanol to dissolve, add 1 kg (11.9 mol) of cyanoacetamide, stir to dissolve, add dropwise hydrogen to oxidize Sodium 80% ethanol solution 6.5L (sodium hydroxide content 1.3kg), temperature control below 0°C; after dropping, stir at -5~0°C for 16h, add 15L of water to the reaction solution, add 500g of activated carbon, stir at room temperature for 20min, filter, Adjust the pH of the filtrate to 7 with dilute hydrochloric acid, filter, wash the filter cake with water until the filtrate is colorless, add 2.2kg of wet dexmilrinone, add 45L of 50vt% ethanol to dissolve under reflux, filter, stir and crystallize the filtrate at -5~0℃ for 6h, filter , and dried at 60-70° C. for 6 hours to obtain 1.86 kg of white crystalline milrinone with a yield of 84.2% and a purity of 99.93% (HPLC method).
Embodiment 3
[0043]Take 2 kg (10.5 mol) of 1-ethoxy-2-(4-pyridyl) vinyl methyl ketone, add 22 L of 60vt% ethanol to dissolve, add 1 kg (11.9 mol) of cyanoacetamide, stir to dissolve, add dropwise hydrogen to oxidize Sodium 80% ethanol solution 6.5L (sodium hydroxide content 1.3kg), temperature control below 0°C; after dropping, stir at -5~0°C for 16h, add 15L of water to the reaction solution, add 500g of activated carbon, stir at room temperature for 20min, filter, Adjust the pH of the filtrate to 7 with dilute hydrochloric acid, filter, wash the filter cake with water until the filtrate is colorless, add 2.1kg of wet dexmilrinone, add 50vt% ethanol 42L to dissolve, filter, stir and crystallize the filtrate at -5~0℃ for 6h, filter , and dried at 60-70° C. for 6 hours to obtain 1.79 kg of white crystalline milrinone, with a yield of 81.0% and a purity of 99.96% (HPLC method).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com