A kind of preparation method of high-purity milrinone
A technology of milrinone and sodium hydroxide, applied in the field of medicine, can solve the problems of relatively high reaction conditions, strict requirements on water for phenyllithium, unsuitable for industrial production, etc., and achieves high product purity, improved production environment, and hydrolysis. The effect of the process is simple and easy to operate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
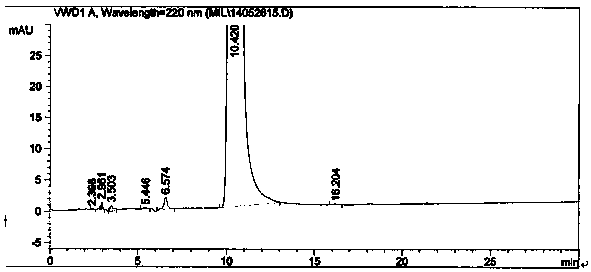
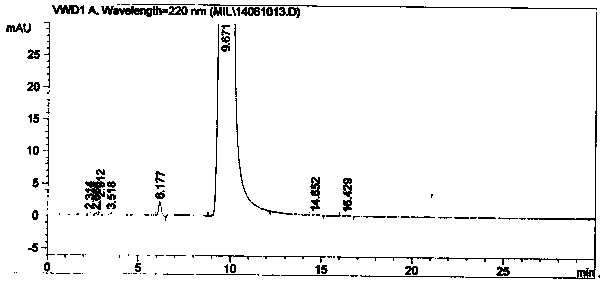
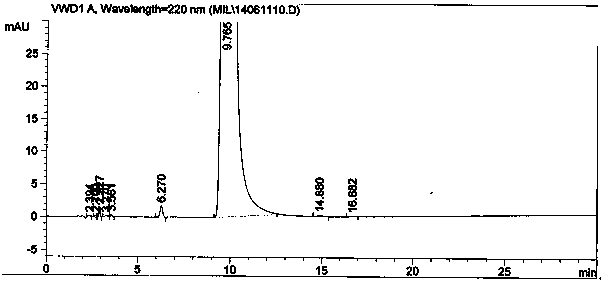
Image
Examples
example 1
[0047] In a 1000mL three-necked flask, add 93.0g (1.0mol) of 4-picoline and 500mL of chloroform, place in an ice-water bath to control the temperature below 50°C, and add 80.0g (1.02mol) of acetyl chloride dropwise. Raise the temperature to 55°C for 2.5 hours. After the reaction is completed, add saturated sodium carbonate aqueous solution dropwise to the system under ice-cooling to adjust the pH to 5-7, then add 30.0g of 30wt% sodium hydroxide solution (sodium hydroxide 0.23mol), and stir at 30-50°C for 2.5hr. After the reaction was completed, the layers were separated, the water layer was removed, and dried over anhydrous sodium sulfate. After the solvent was recovered, the 100-105°C / 217kPa fraction was collected by distillation under reduced pressure, that is, 1-(4-pyridyl)-2-acetone, fraction 97.2g, HPLC: 98.4%, yield 72.08%.
[0048] Add 60.0 (0.44mol) g of 1-(4-pyridyl)-2-propanone into a 500mL round bottom flask, and mix 40.5g (0.44mol) triethyl orthoformate, 92.2g (0....
example 2
[0051] In a 5000mL three-necked flask, add 465.0g (5.0mol) of 4-methylpyridine and 3000mL of chloroform, place in an ice-water bath to control the temperature below 50°C, and add 588.8g (7.5mol) of acetyl chloride dropwise. Raise the temperature to 55°C for 3.5 hours. After the reaction is completed, add saturated sodium carbonate aqueous solution dropwise to the system under ice cooling to adjust the pH to 5-7, then add 160.0 g of 30% sodium hydroxide solution (sodium hydroxide 1.2 mol), and stir at 30-50°C for 2.5 hours. After the reaction was completed, the layers were separated, the water layer was removed, and dried over anhydrous sodium sulfate. After the solvent was recovered, the 100-105°C / 217kPa fraction was collected by distillation under reduced pressure, that is, 1-(4-pyridyl)-2-acetone, fraction 498.2g, HPLC: 98.2%, yield 73.8%.
[0052] Add 280.0g (2.07mol) of 1-(4-pyridyl)-2-propanone into a 3000mL round bottom flask, and mix 572.0g (6.21mol) of triethyl orthof...
example 3
[0055] In a 10000mL three-necked flask, add 930.0 g (10mol) of 4-picoline and 6500mL of chloroform, place in an ice-water bath to control the temperature below 50°C, and add 981.0 g (12.5mol) of acetyl chloride dropwise, and heat up after the dropwise addition React at 55°C for 3.0hr. After the reaction is completed, add saturated sodium carbonate aqueous solution dropwise to the system under ice cooling to adjust the pH to 5-7, then add 315.0 g of 30% sodium hydroxide solution (2.36 mol), and stir at 30-50°C for 3.0 hr. After the reaction was completed, the layers were separated, the water layer was removed, and dried over anhydrous sodium sulfate. After the solvent was recovered, the 100-105°C / 217kPa fraction was collected by distillation under reduced pressure, namely 1-(4-pyridyl)-2-acetone, fraction 996.0g, HPLC: 98.2%, yield 73.8%.
[0056] Add 600.0g (4.44mol) of 1-(4-pyridyl)-2-propanone into a 5000mL round bottom flask, and mix 817.0g (8.87mol) triethyl orthoformate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com