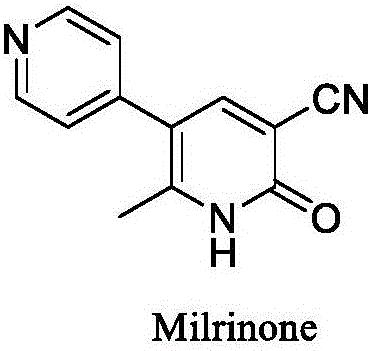
Method for preparing milrinone
A technology of milrinone and compounds, which is applied in the field of preparation of milrinone, can solve the problems of unremovable refining, long reaction time, strict water requirements, etc., so as to save labor costs and raw material costs, facilitate industrial production, and reduce raw material costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
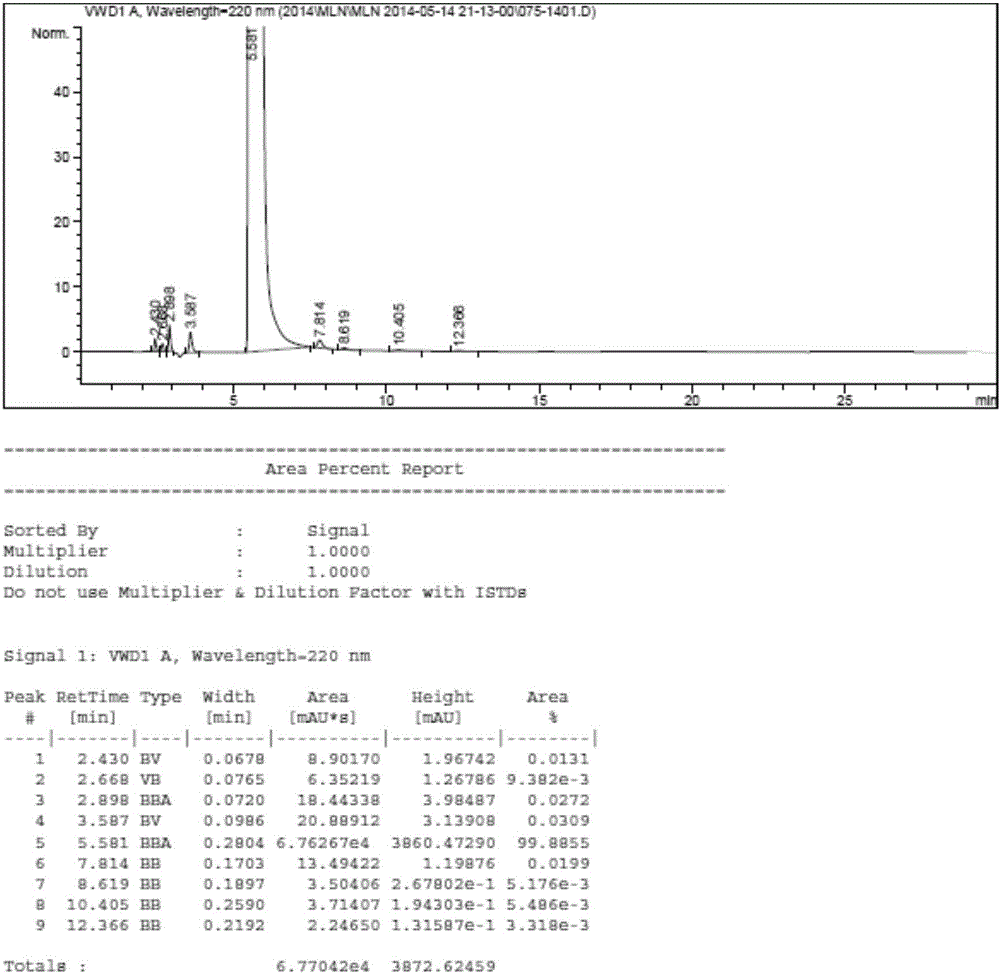
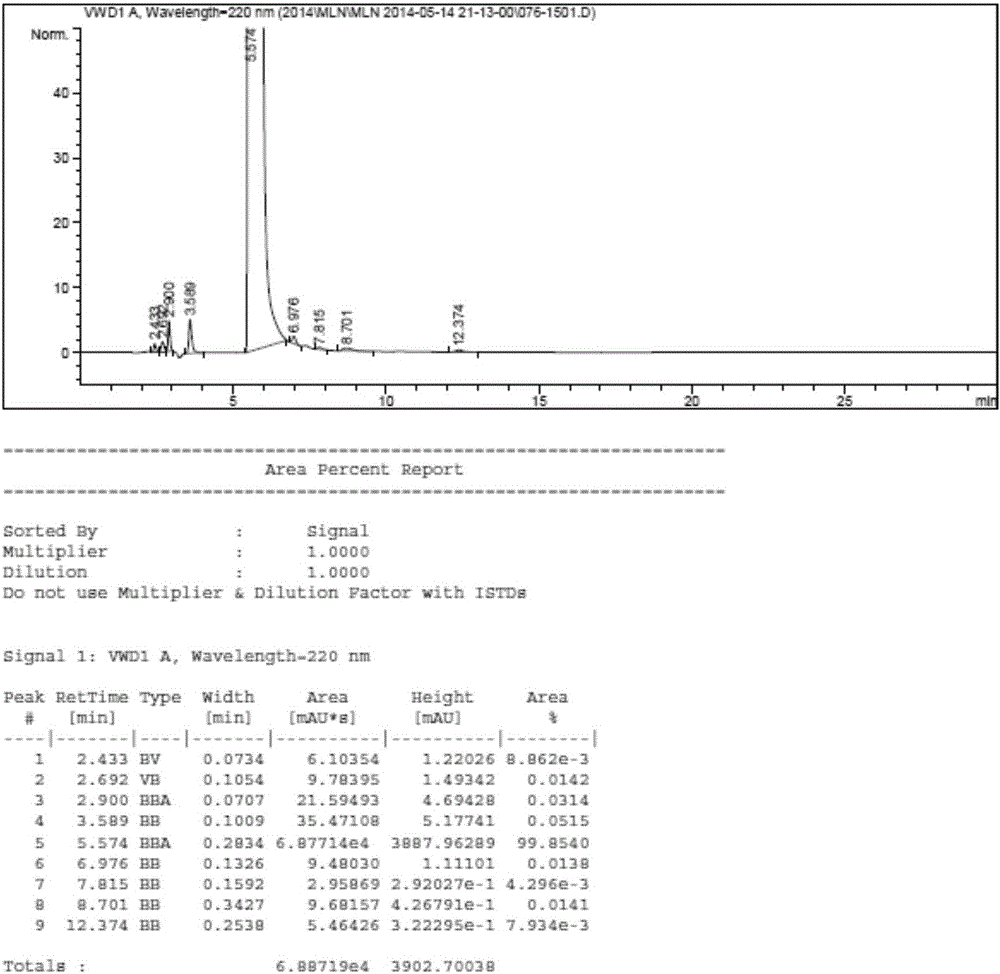
Image
Examples
Embodiment 1
[0032] In the present embodiment, the preparation method of milrinone comprises the following steps:
[0033] 1) Under the protection of nitrogen, add 120ml of tetrahydrofuran and 24ml of diisopropylamine into the reaction flask, cool down to below 20°C, add 74g of 2.0M n-butyllithium solution, and stir at 0-5°C for 20min after the addition is complete. Add 17ml of 4-picoline dropwise, and then add 2ml of hexamethylphosphoric triamide. After the addition is complete, stir at 0-5°C for 1 hour. Then add 25ml of ethyl acetate, heat up to 25°C and stir for 1.5h, cool down to 0°C, add 32ml of hydrochloric acid dropwise, after the drop is complete, concentrate under reduced pressure at 40°C to remove the solvent, add appropriate amount of water and dichloromethane, extract the organic phase, and extract three times , combined organic phases, dried over anhydrous sodium sulfate, filtered, concentrated to remove the organic solvent to obtain a brown liquid, distilled, collected fracti...
Embodiment 2
[0038] In the present embodiment, the preparation method of milrinone comprises the following steps:
[0039] 1) preparing 1-(4-pyridyl)-2-propanone, comprising the following steps:
[0040] Under the protection of nitrogen, add 120ml of ether and 24ml of diisopropylamine into the reaction flask, lower the temperature to below 20°C, add 74g of 2.0M n-butyl lithium solution, after the addition is complete, stir at 0-5°C for 20min. Add 17ml of 4-picoline dropwise, and then add 2ml of hexamethylphosphoric triamide. After the addition is complete, stir at 0-5°C for 1 hour. Then add 25ml of ethyl acetate, heat up to 20°C and stir for 2.5h, cool down to 0°C, add 32ml of hydrochloric acid dropwise, after the drop is complete, concentrate under reduced pressure at 40°C to remove the solvent, add appropriate amount of water and ethyl acetate, extract the organic phase, and extract three times , combined the organic phases, dried over anhydrous sodium sulfate, filtered, concentrated to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com