Preparation method of high-purity milrinone
A milrinone, high-purity technology, used in the field of medicine, which can solve the problems of harsh operating environment and heavy pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
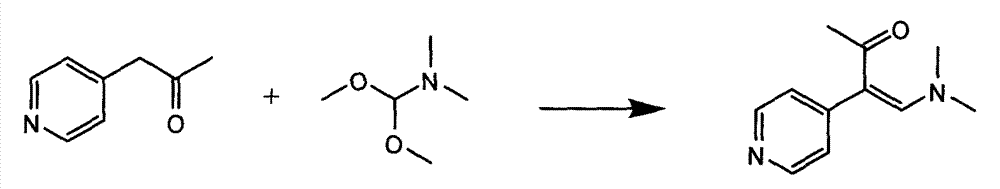
[0027] Add 2.6 kg of 1-(4-pyridyl)acetone and 10.4 kg of N,N-dimethylformamide dimethyl acetal into a 50L reactor, stir, heat to reflux with steam, and reflux for 2 hours. Concentrate under reduced pressure until almost no distillate is evaporated, stop heating, pass circulating water to cool down, and add n-hexane. After cooling to room temperature, stirring was continued for 1 hour. After centrifugation, the obtained solid was dried at 50°C for 4 hours to obtain 3.2 kg of brown solid with a yield of 88.9%.
Embodiment 2
[0029] Add 2.6 kg of 1-(4-pyridyl)acetone, 10.4 kg of N,N-dimethylformamide dimethyl acetal, and 10 kg of acetonitrile into a 50L reactor, stir, heat to reflux with steam, and reflux for 2 hours. Concentrate under reduced pressure until almost no distillate is evaporated, stop heating, pass circulating water to cool down, and add n-hexane. After cooling to room temperature, stirring was continued for 1 hour. After centrifugation, the obtained solid was dried at 50°C for 4 hours to obtain 3.1 kg of brown solid with a yield of 87.3%.
Embodiment 3
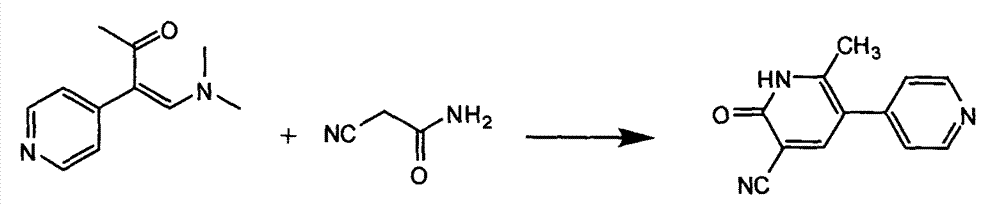
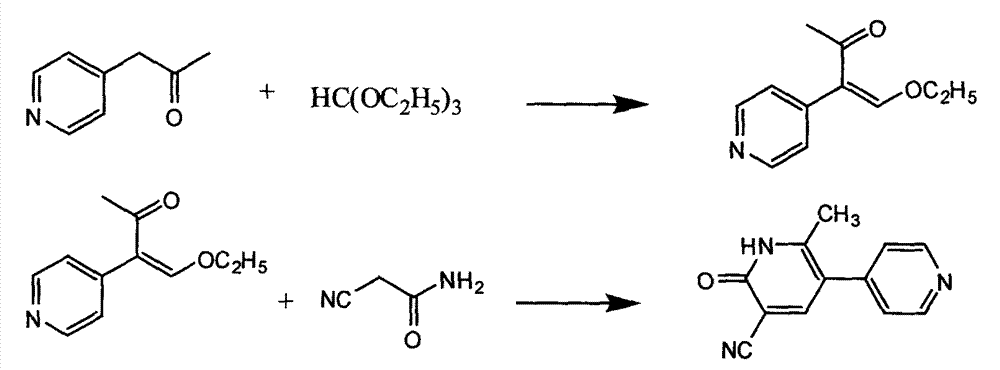
[0031] Add 51.2 kg of methanol, 2.7 kg of sodium methoxide, 3.2 kg of 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone, and 2.8 kg of cyanoacetamide into a 100L reactor, stir and steam Heat to reflux and reflux for 1 hour. Stop heating, lower the temperature to 0-5°C, and continue stirring for 2 hours. Centrifuge, disperse the obtained solid in 10 kg of water, stir, adjust the pH to 2 to 3 with hydrochloric acid, and then adjust the pH to 6 to 7 with about 20% sodium hydroxide solution, and stir at 0 to 5°C for 1 hour. After centrifugation, the obtained solid was dried at 50° C. to obtain 2.1 kg of yellow solid with a yield of 58.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com