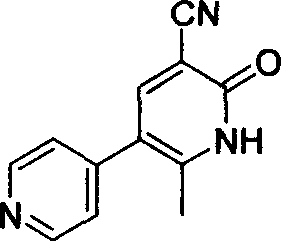
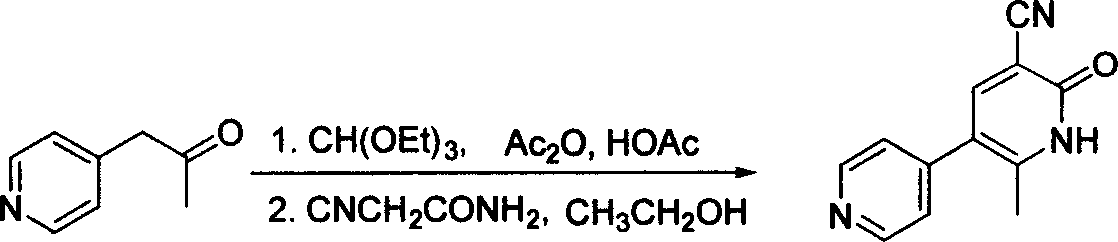Process for preparing milrinone
A synthetic method and technology of acetone, which is applied in the field of preparation of the compound milrinone, can solve the problems of expensive raw materials, complex synthetic lines, and high equipment requirements, and achieve the effects of simplifying operations, simplifying the process, and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0017] Put 147g of 1-(4-pyridyl)-acetone, 235ml of glacial acetic acid, 195ml of acetic anhydride, and 300ml of triethyl orthoformate into a 3-liter three-necked flask, install a thermometer, stir, and continue to react at room temperature for 15 hours, then reduce the pressure After concentrating, add 1500ml of ethanol and 120g of α-cyanoacetamide in sequence, adjust the pH to ≥10 with 2N NaOH aqueous solution at 0°C, filter, soak the filter cake once with ethanol, drain, and dry to obtain 181.2g of crude product, which is used later DMF was recrystallized, and 151.8 g of fine milrinone was collected with a yield of 66%.
example 2
[0019] Put 100g of 1-(4-pyridyl)-acetone, 180ml of glacial acetic acid, 160ml of acetic anhydride, and 195ml of triethyl orthoformate into a 3-liter three-necked flask, install a thermometer, stir, and continue to react at room temperature for 15 hours. After concentrating, add 1000ml of tetrahydrofuran and 90g of α-cyanoacetamide in sequence, adjust the pH to ≥ 11 with 2N NaOH aqueous solution at 0°C, filter, soak the filter cake once with ethanol, drain, and dry to obtain 121g of crude product, which is then washed with DMF After recrystallization, 99.4 g of fine milrinone was collected with a yield of 63.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com