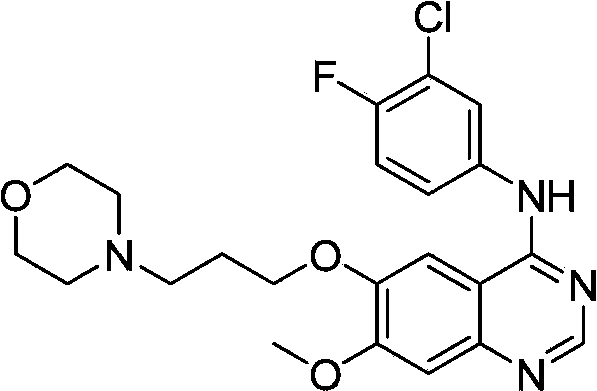
Preparation method of gefitinib
A technology for gefitinib and compounds, applied in the field of pharmaceutical preparation, can solve the problems of low purity and yield of formamidine intermediates, affecting the purity and yield of gefitinib, and difficult separation and purification of products, and achieves a purification method. Simple, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
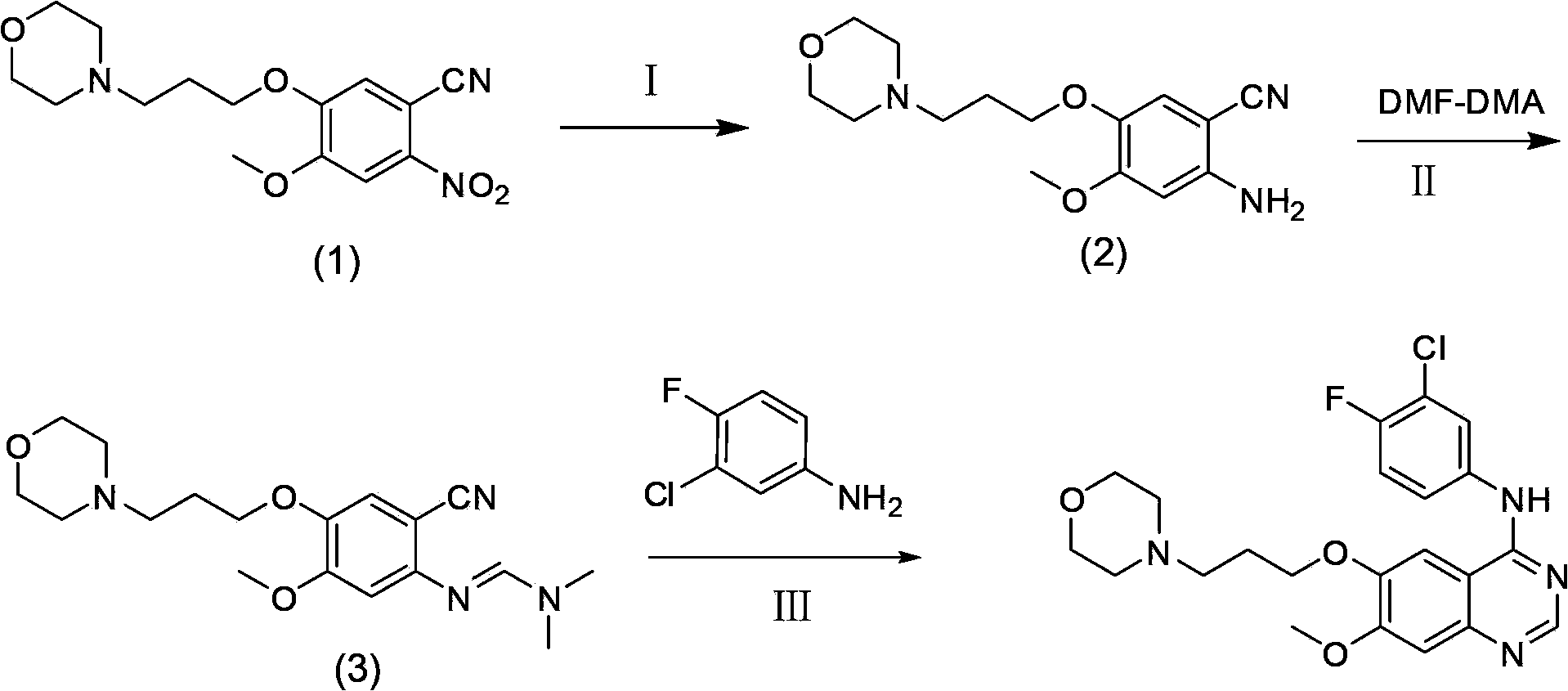
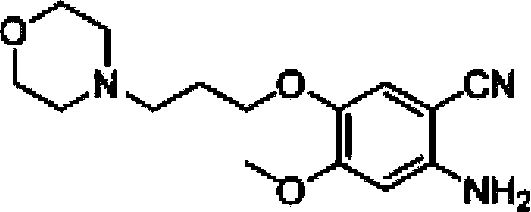
[0030] The invention provides a preparation method of gefitinib: use 4-methoxy-5-(3-morpholine propoxy)-2-nitrobenzonitrile as raw material, and obtain an intermediate through reduction and salt formation 3 reacted directly with N,N-dimethylformamide dimethyl acetal to obtain 5,5 rearranged with 3-chloro-4-fluoroaniline to obtain gefitinib. The synthetic route is as follows:
[0031]
Embodiment 1
[0033] (1) Synthesis of compound 2
[0034] Put 30g of 4-methoxy-5-(3-morpholinopropoxy)-2-nitrobenzonitrile into 400mL of tap water, stir, heat to 50°C, then slowly add 45g of hydrosulfite, keep warm for reaction After 2 hours, the solution was not clear at this time, and the temperature was raised to 70°C, and 151 mL of concentrated hydrochloric acid was added dropwise. After the addition was complete, the solution was clear, and TLC (thin layer chromatography) showed that the reaction of the raw materials was complete. Filter the reaction solution while it is hot (filter out a small amount of insoluble suspended solids), cool the filtrate to room temperature, adjust the pH to 10 with 50% NaOH solution, then extract 500 mL x 3 times with dichloromethane, combine the dichloromethane phases, Washed with water, washed with saturated brine, anhydrous Na 2 SO 4 Drying, rotary evaporation of dichloromethane, and vacuum drying gave 24 g of crude compound 2, with a yield of 88% an...
Embodiment 2
[0043] (1) Synthesis of compound 2
[0044] Put 1500g of compound 1 into a reaction kettle containing 15L of dioxane and 15L of water, stir, heat to 50°C, then slowly add 2440g of sodium hydrosulfite, and keep it warm for 2 hours. At this time, the solution is not clear, and then heated to 70 ℃, add 1500mL of concentrated hydrochloric acid dropwise, after the addition is complete, the solution is clear, and TLC shows that the reaction of the raw materials is complete. Cool the reaction solution to room temperature, add about 1200g of a saturated solution of sodium hydroxide, adjust the pH to 10, then extract 20L x 3 times with dichloromethane, combine the dichloromethane phases, wash with water, wash with saturated saline, and anhydrous Na 2 SO 4 Drying and rotary evaporation of dichloromethane gave 1251 g of the crude product of compound 2 (directly used in the next step of salt formation reaction), with a yield of 92%.
[0045] (2) Synthesis of compound 3
[0046] Dissolv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com