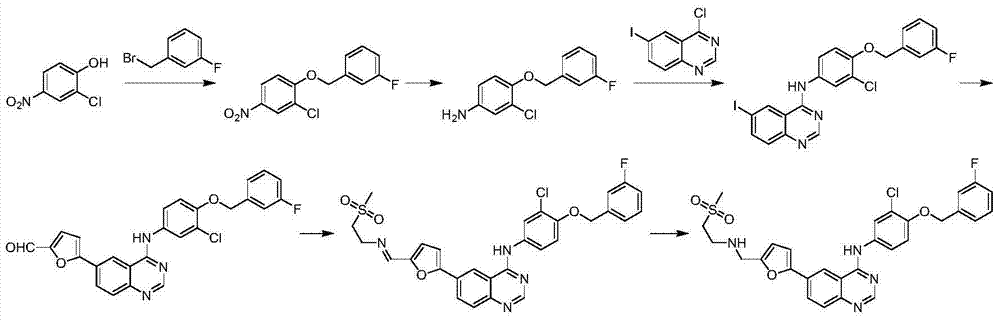
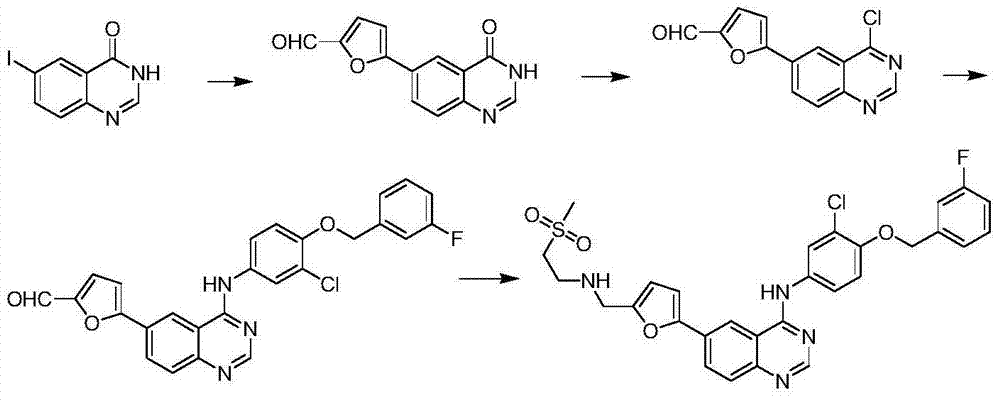
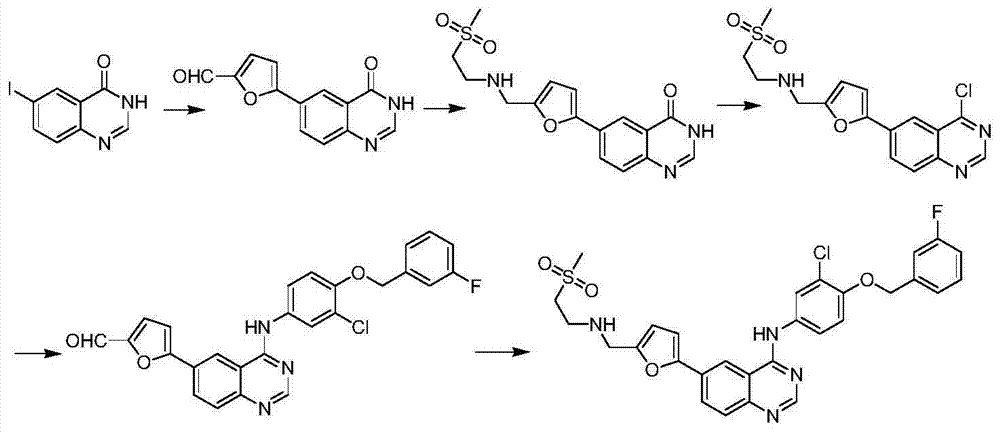
Synthetic method for lapatinib and lapatinib intermediates
A synthesis method and technology of lapatinib, applied in the field of small molecule chemical drug preparation, can solve the problems of difficult post-processing, long reaction route, large environmental pollution, etc., achieve simple and practical synthesis method, shorten synthesis steps, and reduce production cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthetic compound (3)
[0044]
[0045]Dissolve 10g (0.058mol) of compound 1 in 40mL of DMF, add potassium carbonate (8.05g, 0.059mol, 1.3eq), add 8.5g (0.045mol) of compound 2 at 80°C, and react at 95°C for 2 hours , TLC detection, adding 150mL of water, stirring and filtering to obtain 13.5g of bright yellow solid compound 3, yield 83%.
[0046] 1 H-NMR (CDCl 3 ,400MHz, δppm):5.26(s,2H),7.01(d,J=9.2Hz,1H),7.05(t,J=8.4Hz,1H),7.17~7.26(m,2H),7.36~7.40( m, 1H), 8.13 (dd, J=2.8Hz, J=9.2Hz, 1H), 8.33 (d, J=2.4Hz, 1H).
Embodiment 2
[0048] Synthetic compound (4)
[0049]
[0050] Dissolve 1g (3.5mmol) of compound 3 in 17ml of ethanol, add 17ml of acetic acid, add 1.37g (0.024mol, 7eq) of iron powder and a catalytic amount of hydrochloric acid, and react under reflux for 1 hour. After the reaction is complete. Filtered, washed with saturated sodium bicarbonate, evaporated to dryness, and passed through the column to obtain 0.7 g of product compound 4 with a yield of 77%.
[0051] 1 H-NMR (DMSO-d6, 400MHz, δppm): 5.13(s, 2H), 6.84(dd, J=2.4, J=8.8Hz, 1H,), 7.02(d, J=2.4Hz, 1H), 7.16 (t, J=4Hz, 1H), 7.25(s, 1H), 7.31~7.35(m, 1H), 7.42~7.53(m, 2H), 7.8(brs, 2H).
Embodiment 3
[0053] Synthetic compound (6)
[0054]
[0055] Dissolve 10g (0.0169mol) of compound 5 in 100mL of acetic acid, dissolve 14g (0.0172mol) of iodine chloride in 50L of acetic acid, slowly add the above-mentioned iodine chloride acetic acid solution dropwise at room temperature, continue to react for 2h after dropping, and detect by TLC. The reaction liquid was added into ice water, stirred and filtered to obtain a pink solid, which was dried to obtain 14 g of compound 6, with an HPLC purity of 99% and a yield of 70%.
[0056] 1 H-NMR (DMSO-d6, 400MHz, δppm): 6.25(s, 2H), 6.62(d, J=8.8Hz, 1H), 7.54(dd, J=2Hz, J=8.8Hz, 1H), 7.68( d, J=2Hz, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com