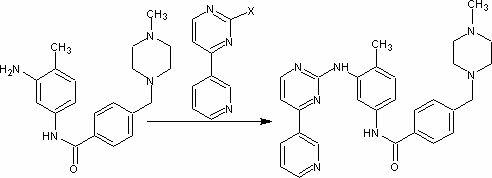
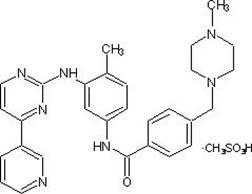
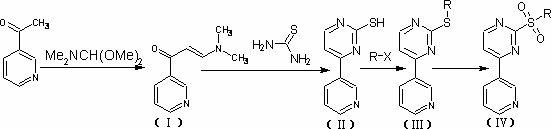Preparation method of imatinib intermediate
A technology of intermediates and compounds, applied in the field of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Synthesis of Methanesulfonyl-4-(3-pyridyl)-pyrimidine
[0044] (1) Synthesis of compound Ⅰ
[0045] Measure 11ML of 3-acetylpyridine into a 250ML single-mouth bottle, then add 27ML of DMF-DMA and mix well, add 35ML of reaction solvent o-xylene, heat to X—Y℃ for reflux reaction; react in reflux state for 13h, cool down to room temperature, add 20ML of n-hexane Placed in the reaction solution, a large amount of light yellow precipitate precipitated out. The solid was collected by filtration to obtain 10.94 g of compound I as a solid product, with a yield of 62.2%.
[0046] ES—MS m / z 177[M+1]
[0047] Melting point: 83.8~84.5℃
[0048] (2) Synthesis of compound Ⅱ
[0049] Weigh 17.6g of solid compound I and 9.13g (0.12mol) of thiourea into a 500ML flask, then add 4g of solid sodium hydroxide and 200ML of n-butanol, and the reaction is carried out under reflux. After reacting for about 1.5 hours, the solvent was evaporated to dryness to obtain 16.2 g of compound II; yi...
Embodiment 2
[0070] Synthesis of Ethyl-4-(3-pyridyl)-pyrimidine
[0071] (1) The synthesis method of compound I and II is the same as in Example 1.
[0072] (2) Synthesis of compound Ⅲ
[0073] Weigh 18.9 g of compound II, dissolve it in 120 mL of sodium hydroxide solution with a concentration of 1 mol / L, add 8 mL of iodoethane, react for about 1 hour, a large amount of precipitation precipitates out, filter, wash with distilled water, and dry to obtain solid compound III. 15.5g, yield 71.4%.
[0074] (3) Synthesis of intermediate IV, namely ethylsulfonyl-4-(3-pyridyl)-pyrimidine
[0075] Weigh 21.5g of solid compound III, dissolve it in 100ML acetone, add 30ML hydrogen peroxide, react at 40°C for 2 hours, evaporate the reaction solution to dryness, dissolve it in dichloromethane, add saturated sodium bisulfite solution, wash three times, and distilled water , the organic phase was dried by adding anhydrous sodium sulfate, and the solvent was evaporated to dryness to obtain 21.8 g of pr...
Embodiment 3
[0084] Synthesis of propanesulfonyl-4-(3-pyridyl)-pyrimidine
[0085] (1) The synthesis method of intermediates I and II is the same as in Example 1.
[0086] (2) Synthesis of compound Ⅲ
[0087] Weigh 18.9 g of compound II, dissolve it in 120 mL of 1 mol / L sodium hydroxide solution, add 8 mL of iodopropane, react for about 1 hour, a large amount of precipitation precipitates out, filter, wash with distilled water, and dry to obtain a solid III compound 17.8 g, yield 77.05%.
[0088] (3) Synthesis of intermediate IV, i.e. propanesulfonyl-4-(3-pyridyl)-pyrimidine
[0089] Weigh 22.7g of solid compound III, dissolve it in 100ML acetone, add 30ML hydrogen peroxide, react at 40°C for 2 hours, evaporate the reaction solution to dryness, dissolve it in dichloromethane, add saturated sodium bisulfite solution, wash three times, and distilled water , the organic phase was dried by adding anhydrous sodium sulfate, and the solvent was evaporated to obtain 23.2 g of product intermedia...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com