Method for detecting afatinib dimaleate isomers and main degradation impurities through high performance liquid chromatography
A technology of high-performance liquid chromatography and maleic acid alpha, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as poor accuracy, poor specificity, and complicated operation, and achieve good accuracy, strong specificity, and sensitivity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: The HPLC detection of the mixed solution of Afatinib maleate and its isomers
[0034] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector;
[0035] Column: ID, 4.6×250mm, 5μm;
[0036] Mobile phase: n-hexane-ethanol-diethylamine (volume ratio 80:20:0.1)
[0037] Detection wavelength: 253nm;
[0038] Flow rate: 0.8mL / min;
[0039] Injection volume: 10μL;
[0040] Column temperature: 30°C;
[0041] Diluent: absolute ethanol;
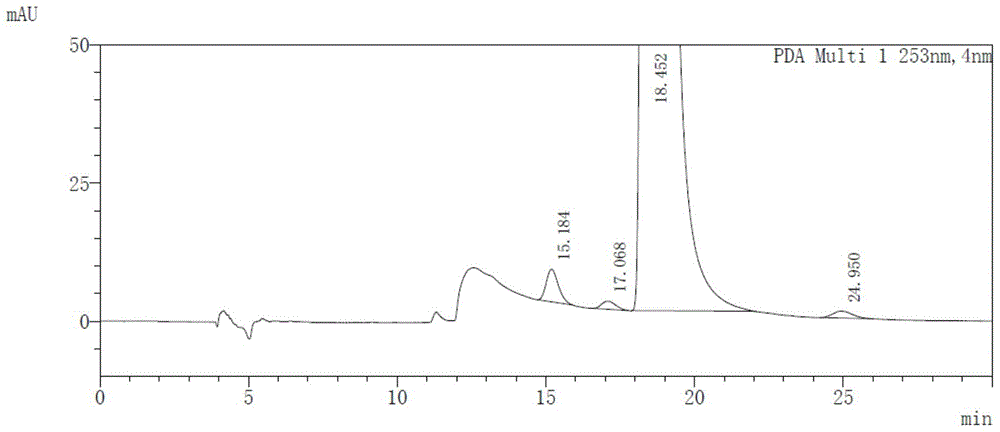
[0042] Take afatinib maleate, impurity D (cis-isomer of afatinib), impurity I and afatinib enantiomer (AFTN-R), dissolve and dilute with absolute ethanol to prepare A mixed solution containing 0.5 mg / ml of afatinib, 2 μg / ml of impurity D, 0.5 μg / ml of impurity I and 0.5 μg / ml of AFTN-R was formed.
[0043] Take blank solvent (diluent) and mixed solution respectively, measure according to the above-mentioned chromatographic conditions, record the chromatogram, the results ...
Embodiment 2
[0045] Embodiment 2: detection of afatinib bulk drug
[0046] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector;
[0047] Column: ID, 4.6×250mm, 5μm;
[0048] Mobile phase: n-hexane-ethanol-ethylenediamine (volume ratio 79:21:0.15)
[0049] Detection wavelength: 253nm;
[0050] Flow rate: 0.8mL / min;
[0051] Injection volume: 10μL;
[0052] Column temperature: 30°C;
[0053] Diluent: absolute ethanol;
[0054] Take an appropriate amount of afatinib maleate raw material, dissolve and dilute with absolute ethanol to prepare a solution containing 0.5 mg / ml of afatinib.
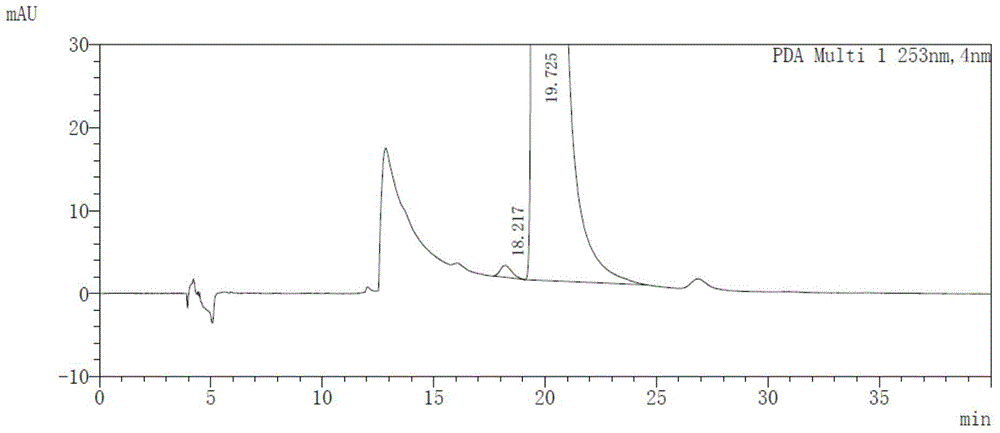
[0055] Get maleic acid afatinib solution, measure according to the above-mentioned chromatographic conditions, record the chromatogram, the results are shown in image 3 .
[0056] image 3 The chromatographic peak whose retention time is 19.725min is the afatinib chromatographic peak. As can be seen from the figure, the optical purity of afatin...
Embodiment 3
[0057] Example 3: Detection of afatinib preparations
[0058] Instrument: Shimadzu LC-20A high performance liquid chromatograph and its workstation VWD (or PDA) detector;
[0059] Column: ID, 4.6×250mm, 5μm;
[0060] Mobile phase: n-hexane-ethanol-triethylamine (volume ratio 81:19:0.05)
[0061] Detection wavelength: 253nm;
[0062] Flow rate: 0.8mL / min;
[0063] Injection volume: 10μL;
[0064] Column temperature: 30°C;
[0065] Diluent: absolute ethanol;
[0066] Take an appropriate amount of fine powder of afatinib maleate preparation, approximately equivalent to 25mg of afatinib, put it in a 50mL measuring bottle, add an appropriate amount of diluent and sonicate for 10min, add diluent to make up to the mark, shake well, filter, Take the continued filtrate as the test solution. Prepare the blank excipient solution in the same way.
[0067] Take blank auxiliary material solution and need testing solution respectively, measure according to above-mentioned chromatog...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com