High purity preparation method of Afatinib intermediate
A compound and reaction technology, which is applied in the field of preparation of -N4--7-oxyl)-quinazoline-4,6-diamine, can solve the problems of difficult industrialization, high purity of dehalogenated impurities, and post-processing troubles and other problems, to achieve the effect of convenient preparation, lower production cost, and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add the compound of formula (III) (50g) into acetonitrile (200ml), slowly add phosphorus oxychloride (41.25g), then drop into triethylamine (27g), heat to reflux, and maintain reflux for 5h, drop into formula ( IV) A mixed solution of the compound (38.75 g) dissolved in 1,4-dioxane (250 ml). After dripping, continue to reflux for 1 hour, cool down to room temperature and add water (200ml), adjust the pH to 7-8 with 5N sodium hydroxide solution, stir for 1 hour, filter, wash the filter cake with water (200ml×2), drain, and solid After drying in a blast oven at 50°C for 12 hours, 72 g of the compound of the yellow formula (V) was obtained.
Embodiment 2
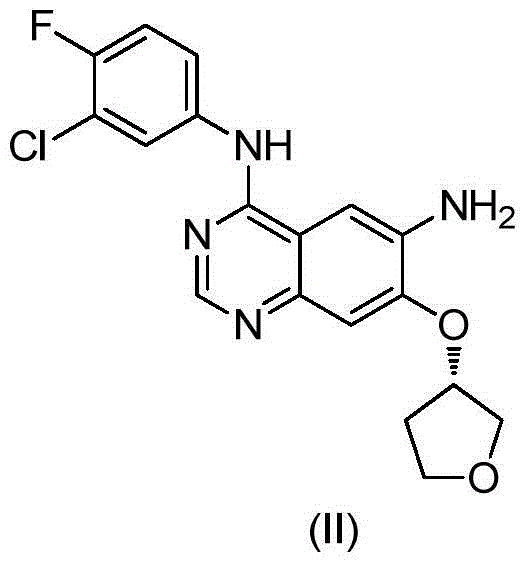
[0032] Add the compound of formula (VI) (13.6g) into DMF (385ml), stir in an ice bath and add potassium tert-butoxide (48g) in batches. And react at room temperature for 2 hours, slowly add water (2L), adjust the pH to 7-8 with 2N hydrochloric acid solution, stir at room temperature for 1 hour, filter, and dry the solid in a blast oven at 50°C for 12 hours to obtain 47g of a yellow compound of formula (VII).
Embodiment 3
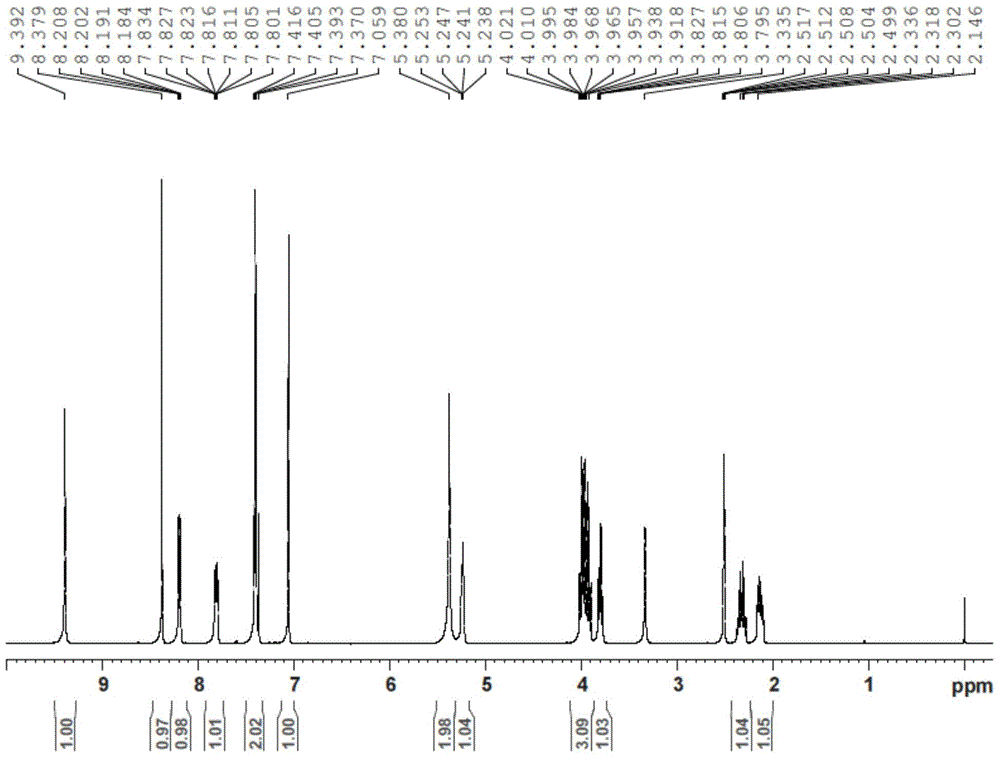
[0034] Mix 1.5L THF, 100g of the compound of formula (VII), 10g of activated carbon, FeCl 3 1g and 100g of hydrazine hydrate were poured into the reaction flask in turn, refluxed for 3 hours, filtered, the filtrate was stirred at room temperature for 4 hours, filtered, and the solid was dried in a blast oven at 50°C. 88 g of off-white solid was obtained, the molar yield was 95%, and the HPLC was 99.57%. The structure confirmation map of the obtained final product is as follows: figure 1 shown.
[0035] 1 H-NMR(DMSO-d6)δ:2.13(m,1H),2.33(m,1H),3.80(m,1H),3.96(m,3H),5.24(br,1H),5.38(s,2H ),7.06(s,1H),7.39(s,1H),7.81(m,1H),8.20(m,1H),8.38(s,1H),9.39(s,1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com