Compound, and preparation method and application thereof
A compound, afatinib technology, applied in the field of afatinib degradation of impurities and its preparation, to achieve the effect of simple reaction operation process, easy control of process operation, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
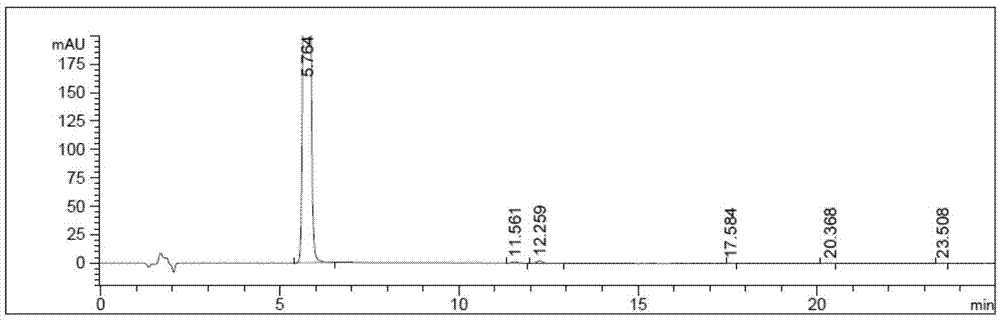
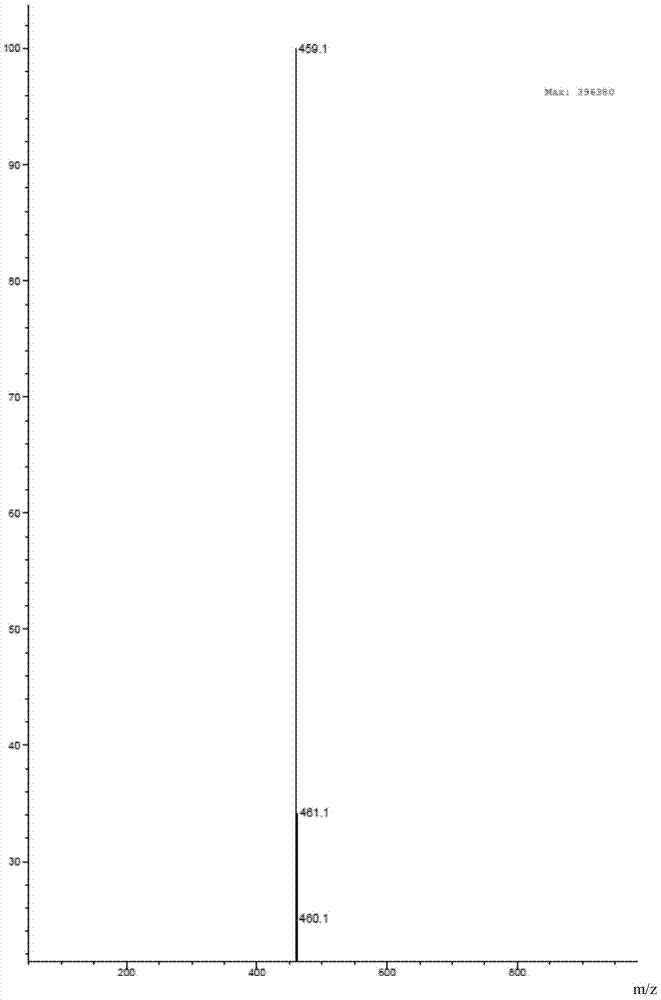
Image
Examples
Embodiment 1
[0041] The steps of synthetic compound 5 are as follows:
[0042] (1) Add 125.1g of compound 4(S)-3-hydroxytetrahydrofuran and 1200ml of DMF to the three-necked flask, start stirring, cool down to 0°C in an ice-water bath, add 160g of potassium tert-butoxide, and stir for 1 hour;
[0043] (2) Add 120 g of compound 3 (N-(3-chloro-4-fluorophenyl)-7-fluoro-6-nitro-4-quinazolinamine) and react at room temperature for 2-6 hours.
[0044] (3) Take a sample, quench the central control with water, pour the reaction solution into water, adjust the pH to 7 with hydrochloric acid, filter, wash with water, and dry to obtain 137 g of compound 5 with a yield of 95%.
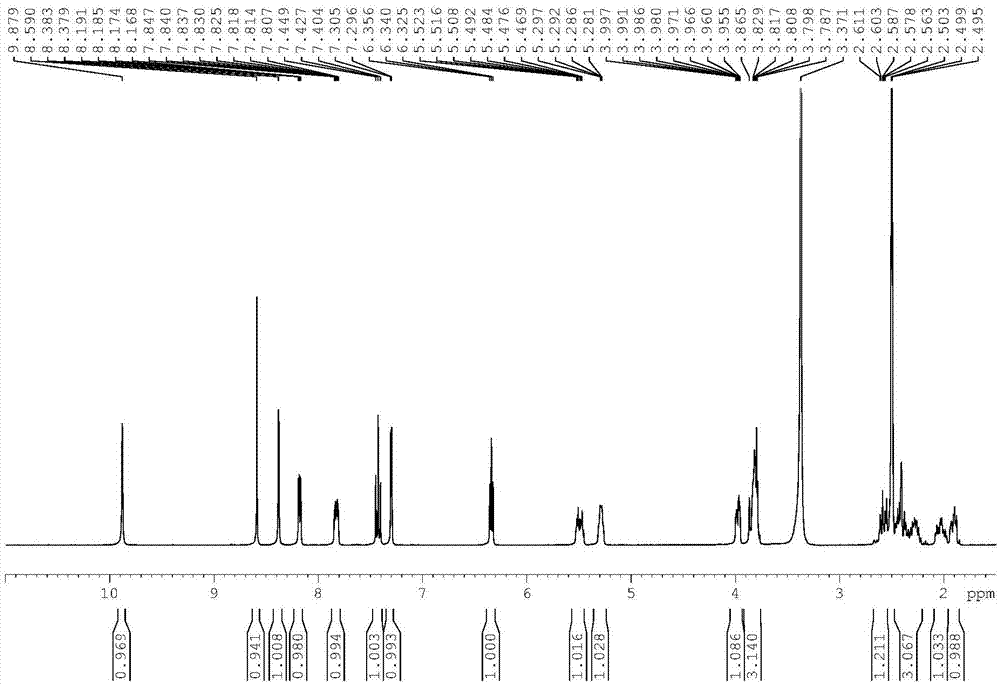
[0045] The H NMR spectrum data of compound 5: 1 H-NMR (DMSO-d 6), 5.42(s,1H),7.42-7.46(t,J=10.8Hz,2H),7.77-7.79(t,J=2.8Hz,1H),8.13-8.14(d,J=4.8Hz,1H),8.65 (s,1H),9.20(s,1H),10.14(s,1H).
Embodiment 2
[0047] The steps of synthetic compound 6 are as follows:
[0048] (1) Add 100g of compound 5 to the three-necked flask: (4-[(3-chloro-4-fluorophenyl)amino]-6-nitro-7-((S)-tetrahydrofuran-3-yloxy) -quinazoline), 2500mL ethyl acetate, add 500ml glacial acetic acid, 500ml water, 56g iron powder under stirring, heat up to 70°C for 1h;
[0049] (2) complete reaction, cooling and filtering, the filter cake is washed with ethyl acetate, the filtrate is allowed to stand for stratification, the water phase is retained, and the organic phase is placed;
[0050] (3) Add ethyl acetate to the aqueous phase, extract and separate the liquids, and combine the organic phases;
[0051] (4) Add 2L of water to the organic phase, adjust the pH to 8 with sodium hydroxide solution under cooling, let stand for stratification, wash with water, wash with saline, stand for stratification, dry and filter with anhydrous sodium sulfate. The filtrate was concentrated to dryness to obtain 84 g of compound ...
Embodiment 3
[0053] The steps of synthetic compound 8 are as follows:
[0054] (1) Add 350mL of tetrahydrofuran into the three-necked reaction flask, start stirring, and add 54.3g of 1,1-dicarbonylimidazole to form a white suspension. Raise the temperature to 40°C, dissolve 57.1g of diethylphosphonoacetic acid in tetrahydrofuran, add dropwise to the system, and stir at 40°C for 30-45min. Above-mentioned reaction mixture is solution A;
[0055] (2) Add 420 mL of tetrahydrofuran into the three-necked reaction flask, start stirring, and add 84.0 g of compound 6, which is a pale green solution. Raise the temperature to 40°C and the above solution is B, add the above solution A to the solution B dropwise, after the addition, the internal temperature drops to 30°C to react;
[0056] (3) Stir for 2 hours, and the reaction is complete. Add 420 mL of methyl tert-butyl ether and cool to room temperature. After stirring continuously for 30 minutes, the above white suspension is suction-filtered. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com