EGFR inhibitor for targetedly treating cancer and preparation method and application thereof
A cancer and oxidant technology, applied in organic chemistry, drug combination, antineoplastic drugs, etc., to achieve the effect of small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
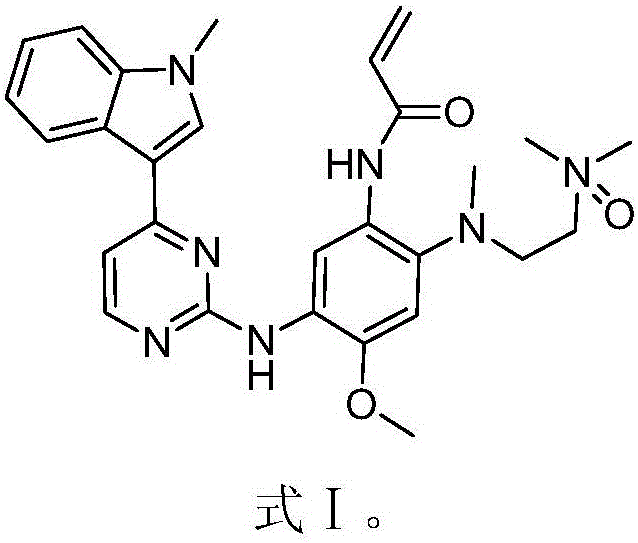
[0029] The preparation of the compound shown in embodiment 1, formula I
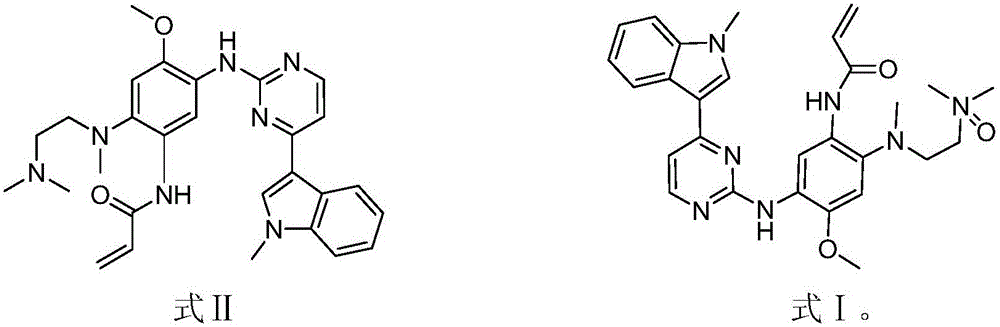
[0030] Add 81 mg of m-chloroperoxybenzoic acid to a solution of AZD9291 (formula II, 200 mg) in dichloromethane (25 mL) under stirring condition. Stir at 20 °C for 30 min. After the reaction was detected by TLC, the reaction solution was washed with saturated sodium bicarbonate solution (25 mL), extracted three times with dichloromethane (25 mL×3), and the obtained organic solvent layers were combined together, and the organic solvent was removed on a rotary evaporator. Purified by reverse HPLC (CH 3 OH / H 2 O10:1), to obtain the compound shown in formula I.
[0031] 1 H-NMR (400MHz, DMSO-d6) δ (ppm) 13.21 (s, 1H), 8.86 (s, 1H), 8.50 (s, 1H), 8.31 (d, J = 5.32Hz, 1H), 8.26 (d ,J=7.88Hz,1H),7.99-7.97(m,3H),7.50(J=8.40Hz,1H),7.26-7.14(m,4H),6.97(s,1H),6.20-6.15(m, 1H),5.62-5.59(m,1H),3.89(s,3H),3.86(s,3H),3.46-3.44(m,2H),3.33(s,3H),3.30(s,3H),3.25 -3.24(m,2H),2.74(s,3H);LRMS(ESI)calcdforC 16 h 18 C...
Embodiment 2
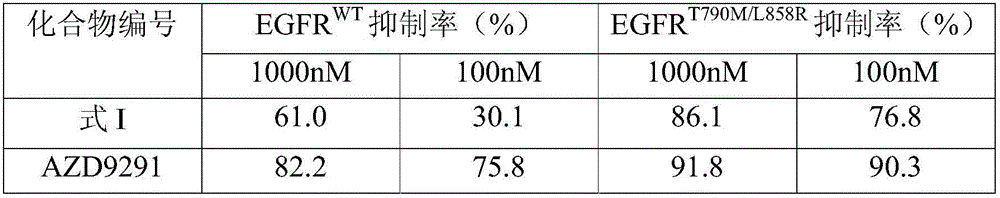
[0032] The inhibitory activity of the compound shown in embodiment 2, formula I to kinase
[0033] 1. Kinase test
[0034] The following methods are used to measure the effect of the compounds of the present invention on EGFR WT and EGFR T790M / L858R Inhibition of enzyme activity, the specific method is as follows:
[0035] The models of the kits are shown in Table 1.
[0036] Table 1 Kinase Model
[0037] Kinase
[0038] Invitrogen kits were used to detect the in vitro activity of EGFR inhibitors. According to the instructions of the kit, prepare the corresponding concentrations of enzyme buffer, enzyme / substrate peptide solution, ATP solution and fully phosphorylated substrate peptide in sequence, and mix gently; use distilled water to prepare a solution of the compound to be tested at a concentration of 4X ,well mixed.
[0039]Add 2.5 μL of the prepared enzyme / substrate peptide solution and fully phosphorylated substrate peptide into a 384-well plate, then ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com