Afatinib-maleate crystal form, and preparation method and pharmaceutical compositions thereof
A technology of maleate and afatinib, which is applied in the field of afatinib-maleate crystal form and its preparation, can solve the problems of not providing detection data comparison data, not providing beneficial properties, etc., to achieve Good thermal stability and storage stability, high crystallinity, good solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0074] For the preparation of the prior art crystal form of afatinib dimaleate, refer to Example 3 of patent document CN1867564B. The specific operation is as follows:
[0075] Take 1.0 g of afatinib free base, add 14 mL of ethanol and stir to dissolve, and heat to 70°C. Take 0.5g of maleic acid, add 6mL of ethanol and stir to dissolve, slowly add the ethanol solution of maleic acid dropwise to the ethanol solution of afatinib free base and stir. After the solid precipitates, the reaction solution is cooled to 20°C and stirred for 2 hours Then, it was stirred at 0°C for 3 hours, filtered, washed with ethanol, and the solid was vacuum dried at 40°C overnight to obtain afatinib dimaleate with a yield of 90%.
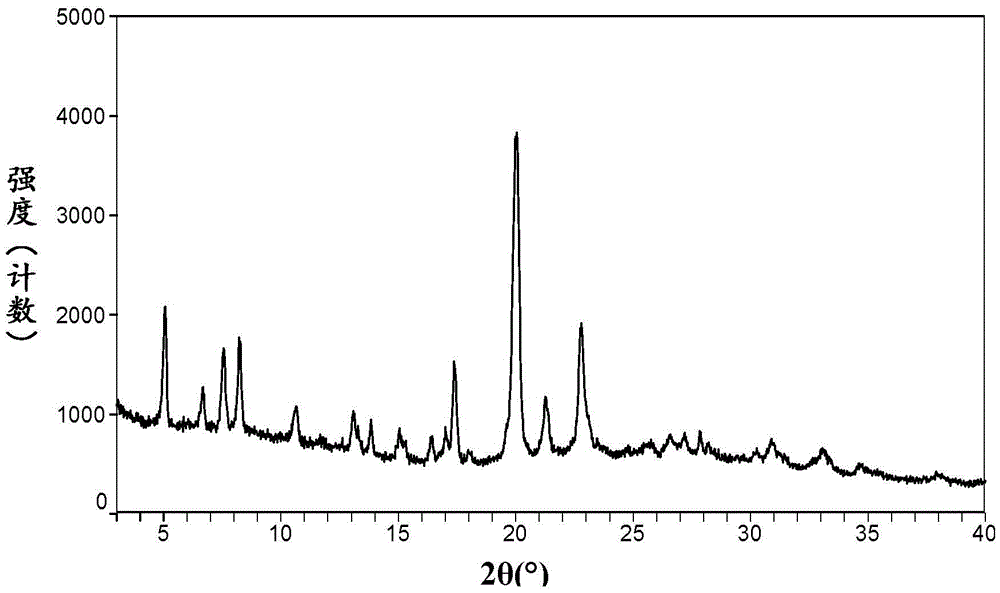
[0076] Its XRPD map is as follows figure 1 As shown, the display is consistent with the prior art crystal form of afatinib dimaleate disclosed in CN1867564B.
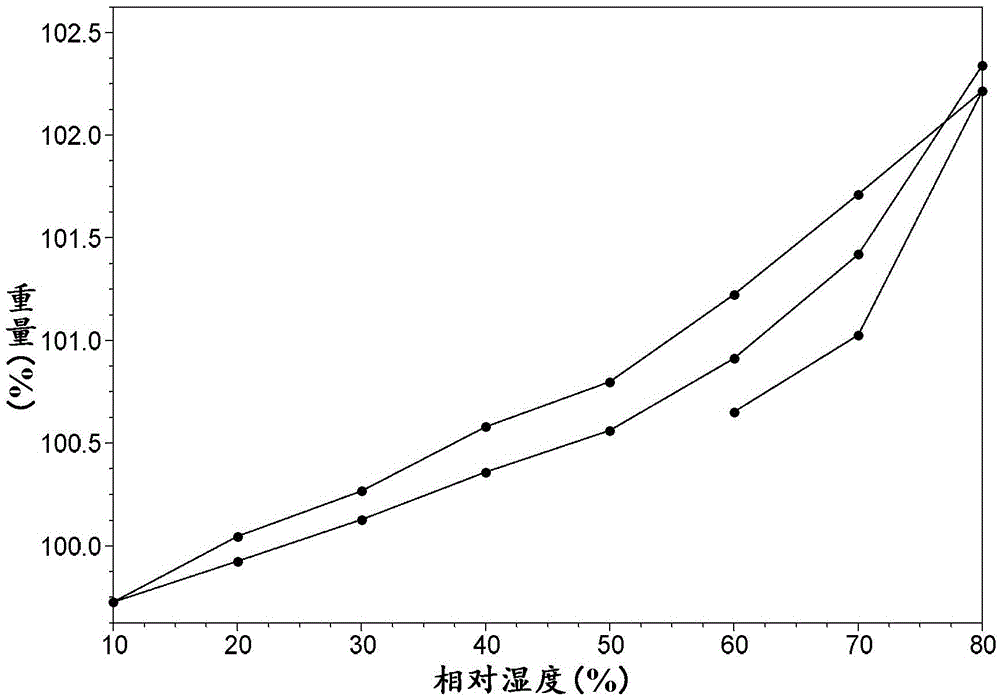
[0077] Its DVS isotherm adsorption curve is as figure 2 As shown, it is shown that the weight change of the salt is 2...
Embodiment 1
[0079] Example 1 Preparation of afatinib maleate crystal form N
[0080] At room temperature, take 5.0 g of afatinib free base, add 20 mL of tetrahydrofuran and dissolve it ultrasonically, add 1.79 g of maleic acid to the tetrahydrofuran solution of afatinib free base to form a solution and stir, slowly add 60 mL of methyl tert The butyl ether was formed into a slurry, stirred for 1 day to crystallize, filtered, and the filter cake was vacuum dried at 40° C. for 8 hours to obtain 5.3 g of afatinib monomaleate crystal form N with a yield of 85.6%.
[0081] 1 H-NMR (DMSO) data are as follows: 9.93(s,1H), 9.77(s,1H), 8.95(s,1H), 8.57(s,1H), 8.05-8.15(m,1H), 7.72-7.85( m,1H),7.44(t,J=9.0Hz,1H),7.28(s,1H),6.81(s,1H),6.10(s,1H),5.32(s,1H),3.85-4.05(m ,5H),3.71-3.85(m,1H),2.75-2.85(m,1H), 2.83(s,6H), 2.30-2.42(m,1H), 2.06-2.20(m,1H). It shows that the ratio of afatinib free base to maleic acid in the salt is about 1:1.
[0082] HPLC detection showed that the content of afatinib in the s...
Embodiment 2
[0086] Example 2 Preparation of afatinib maleate crystal form N
[0087] At room temperature, take 100 mg of afatinib free base, add 0.5 mL of ethanol to dissolve it ultrasonically, add 24 mg of maleic acid to the ethanol solution of afatinib free base to form a solution and stir, add 2.5 mL of n-heptane, and stir After 2 days of crystallization, filtration, and vacuum drying at 40°C for 16 hours, 105 mg of afatinib maleate form N was obtained, with a yield of 84.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com