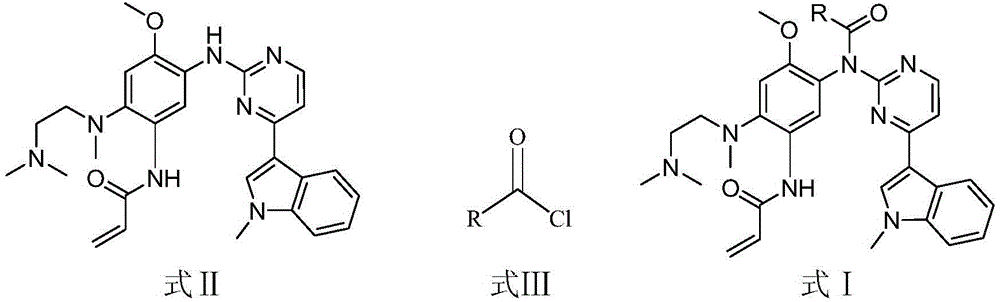
EGFR (epidermal growth factor receptor) inhibitor for targeted therapy of cancers, and preparation method and application thereof
A cancer, condensation reaction technology, applied in the field of EGFR inhibitors, to achieve the effect of small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
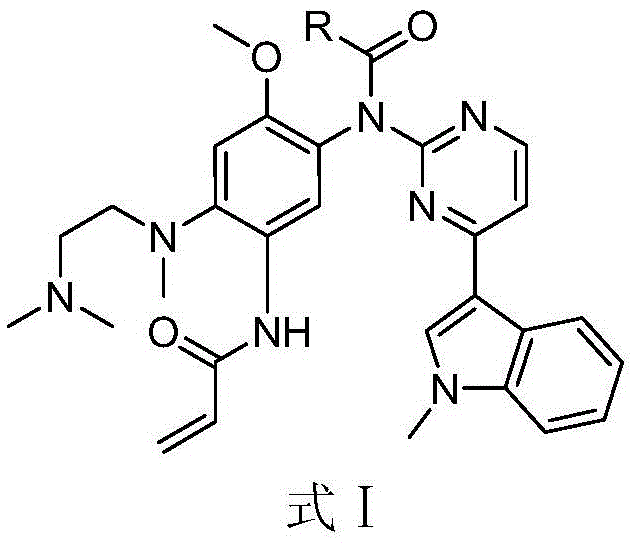
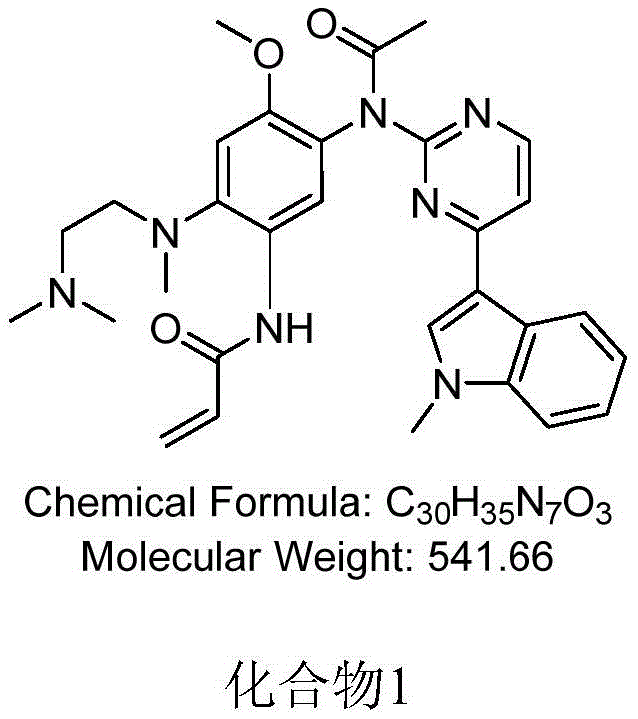
[0033] Embodiment 1, the preparation of compound 1
[0034]
[0035] Add 16 μL of acetyl chloride (the molar ratio of AZD9291 to acetyl chloride is 1:1.1) and 69 μL of N,N-diiso Propylethylamine. Stir at 20 °C for 30 min. After the reaction was detected by TLC, the reaction solution was washed with saturated sodium bicarbonate solution (25 mL), extracted three times with dichloromethane (25 mL×3), and the obtained organic solvent layers were combined together, and the organic solvent was removed on a rotary evaporator. Purification by silica gel column (methanol / dichloromethane 1:30) gave compound 1.
[0036] 1 H-NMR (400MHz, DMSO-d6) δ (ppm) 10.01 (s, 1H), 8.52 (d, J = 5.2Hz, 1H), 8.38 (s, 1H), 8.15 (s, 1H), 7.84 (d ,J=8.0Hz,1H),7.53(d,J=5.2Hz,1H),7.47(d,J=8.0Hz,1H),7.19(t,J=8.0Hz,1H),7.11(s,1H ), 7.00(t, J=8.0Hz, 1H), 6.39-6.36(m, 1H), 6.18(d, J=16.8Hz, 1H), 5.72(d, J=11.6Hz, 1H), 3.85(s ,3H),3.71(s,3H),2.92(s,2H),2.76(s,3H),2.40(s,4H),2.21(s,6H);LRMS(ESI)calcdforC ...
Embodiment 2
[0037] Embodiment 2, the preparation of compound 2
[0038]
[0039]Add 19 μL of propionyl chloride (the molar ratio of AZD9291 to propionyl chloride is 1:1.1) and 69 μL of N,N-diiso Propylethylamine. Stir at 25 °C for 30 min. After the reaction was detected by TLC, the reaction solution was washed with saturated sodium bicarbonate solution (25 mL), extracted three times with dichloromethane (25 mL×3), and the obtained organic solvent layers were combined together, and the organic solvent was removed on a rotary evaporator. Purification on a silica gel column (methanol / dichloromethane=1:30) gave compound 2.
[0040] 1 H-NMR (400MHz, DMSO-d6) δ (ppm) 9.94 (s, 1H), 8.50 (d, J = 5.12Hz, 1H), 8.36 (s, 1H), 8.10 (s, 1H), 7.84 (d ,J=7.68Hz,1H),7.53-7.45(m,2H),7.20-7.16(m,1H),7.07(s,1H),7.00-6.97(m,1H),6.48(br,1H), 6.20-6.15(m,1H),5.72-5.70(m,1H),3.84(s,3H),3.71(s,3H),3.00(br,2H),2.75-2.73(m,5H),2.28( br,6H),1.08(t,J=7.88Hz,3H);LRMS(ESI)calcdforC 16 h 18 Cl 2 N 2 O[M+H] ...
Embodiment 3
[0041] Embodiment 3, the preparation of compound 3
[0042]
[0043] Add 18 μL acryloyl chloride (the molar ratio of AZD9291 to acryloyl chloride is 1:1.1) and 69 μL N,N-diisopropyl Ethylamine. Stir at room temperature for 30 min. After the reaction was detected by TLC, the reaction solution was washed with saturated sodium bicarbonate solution (25 mL), extracted three times with dichloromethane (25 mL×3), and the obtained organic solvent layers were combined together, and the organic solvent was removed on a rotary evaporator. Purification by silica gel column (methanol / dichloromethane 1:30) gave compound 3.
[0044] 1 H-NMR (400MHz, DMSO-d6) δ (ppm) 10.03 (s, 1H), 8.49 (d, J = 5.48Hz, 1H), 8.37 (s, 1H), 8.17 (s, 1H), 7.54 (d ,J=5.48Hz,1H),7.47(d,J=8.24Hz,1H),7.19(t,J=7.60Hz,1H),7.09(s,1H),6.97(t,J=7.60Hz,1H ),6.79-6.73(m,1H),6.44-6.36(m,,1H),7.06(s,1H),6.29(dd,J=16.8Hz,J=1.6Hz,1H),6.17(dd,J =16.8Hz, J=1.6Hz,1H),5.75-5.70(m,2H),3.85(s,3H),3.38(s,3H),2,76(br,2H),2.76(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com