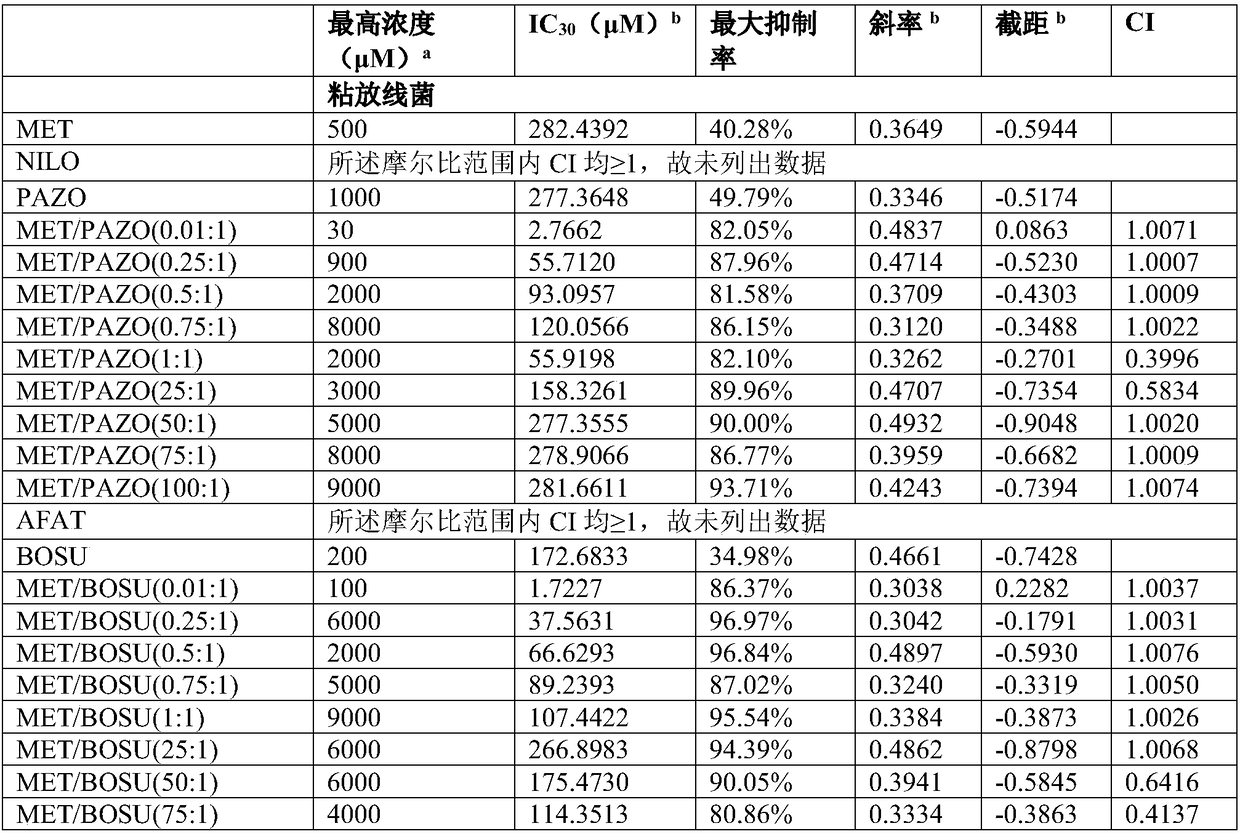
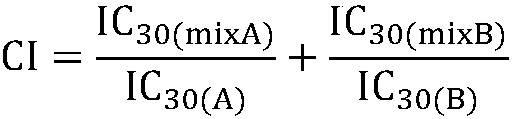Composition containing protein kinase inhibitor and metformin
A protein kinase inhibitor, metformin technology, applied in the field of compositions containing protein kinase inhibitor and metformin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 comprises the preparation of the capsule of metformin hydrochloride and nilotinib hydrochloride monohydrate (molar ratio is 0.01:1)
[0054] prescription
[0055]
[0056] Preparation
[0057] Take the prescribed amount of metformin hydrochloride, nilotinib hydrochloride monohydrate, sorbitol and sodium stearyl fumarate, fully mix, fill in capsules, and subpackage.
Embodiment 2
[0058] Embodiment 2 comprises the preparation of the tablet of metformin hydrochloride and pazopanib hydrochloride (molar ratio is 0.5:1)
[0059] prescription
[0060]
[0061]
[0062] Preparation
[0063] Get the prescribed amount of metformin hydrochloride, pazopanib hydrochloride, microcrystalline cellulose, magnesium stearate, fully mix, and compress into tablets.
Embodiment 3
[0064] Example 3 Preparation of granules comprising metformin hydrochloride and afatinib dimaleate (molar ratio of 1:1)
[0065] prescription
[0066]
[0067] Preparation
[0068] Take the prescribed amount of metformin hydrochloride, afatinib dimaleate, microcrystalline cellulose, and sodium stearyl fumarate, mix them thoroughly, and pack them separately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com