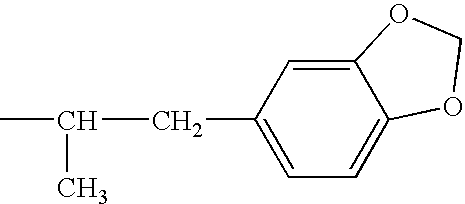
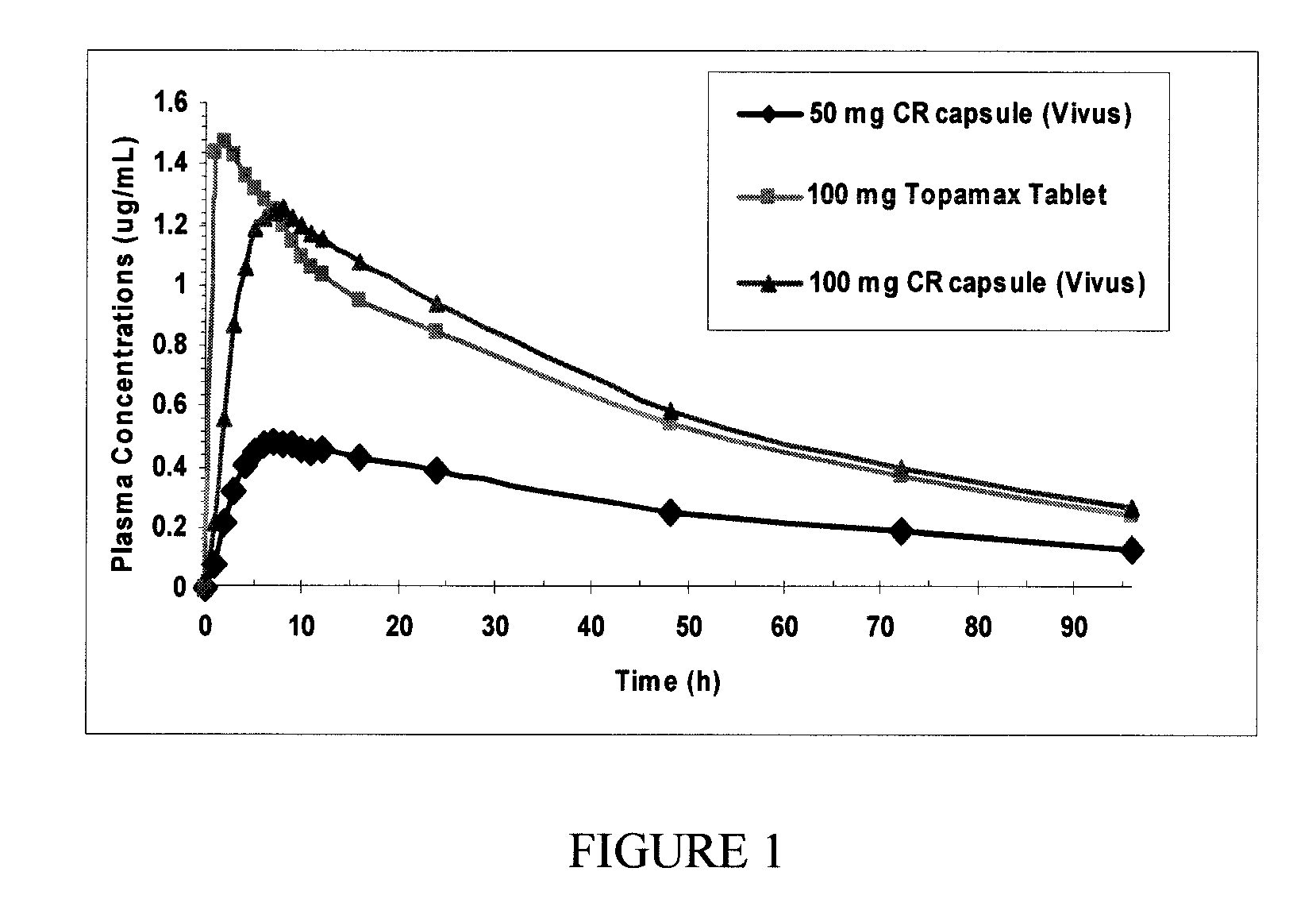
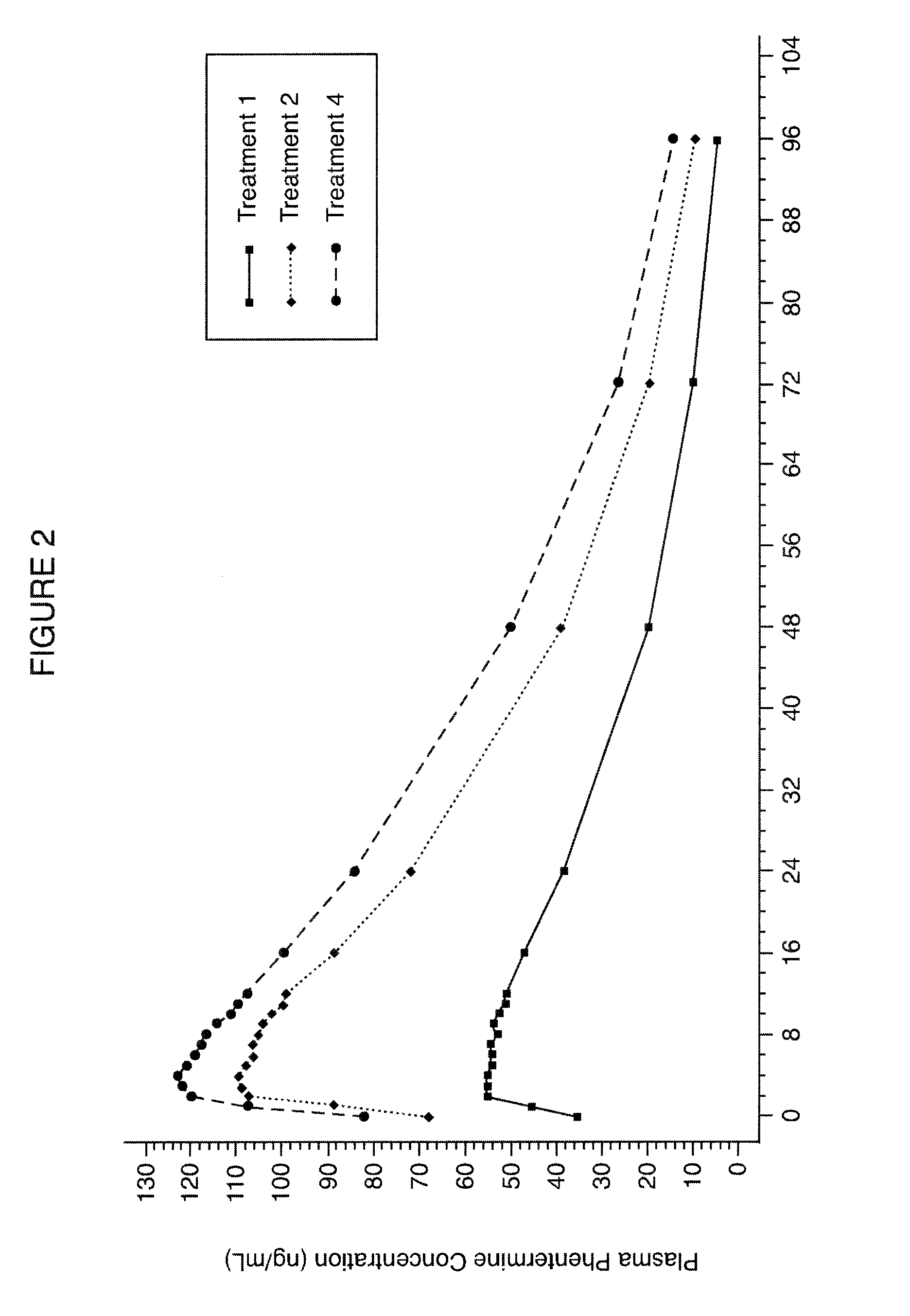
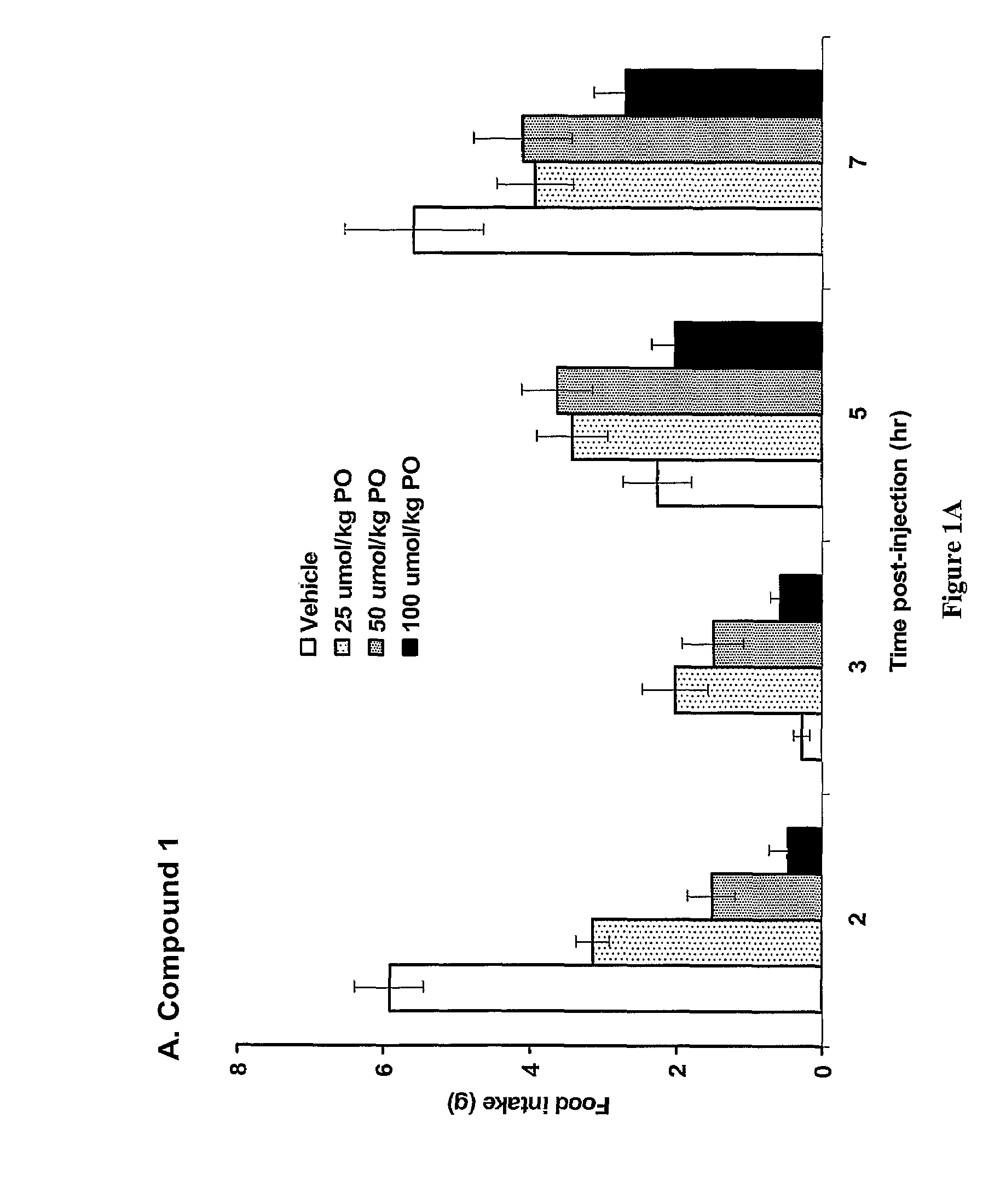
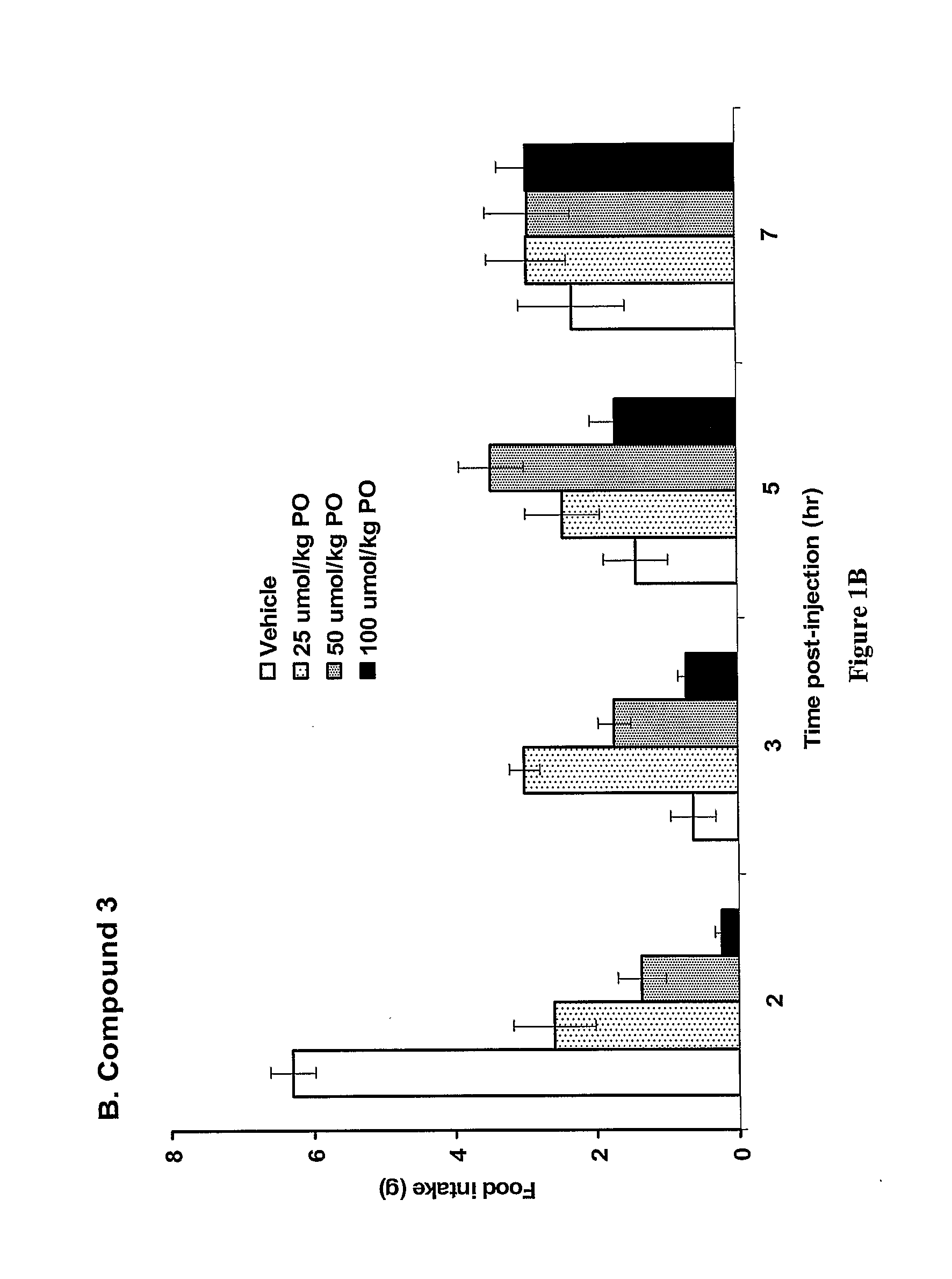
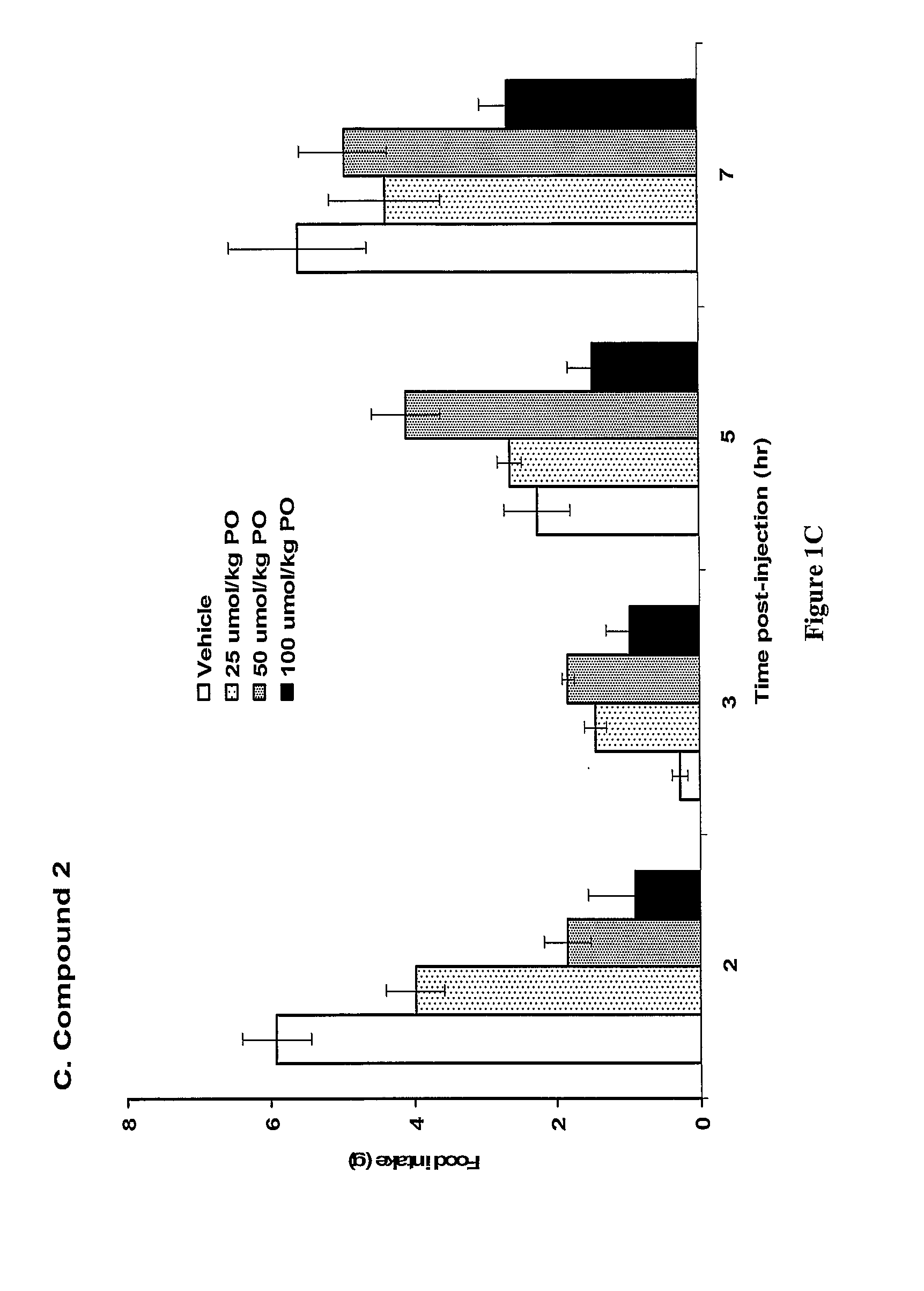
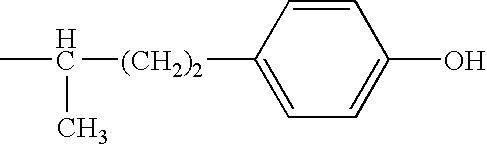Patents
Literature
42 results about "Phentermine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
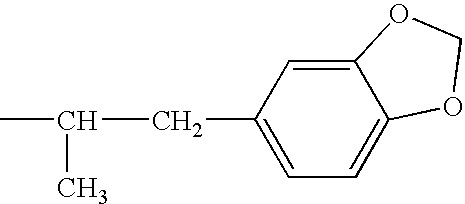
Phentermine is used with a doctor-approved exercise, behavior change, and reduced-calorie diet program to help you lose weight. It is used by certain overweight people, such as those who are obese or have weight-related medical problems.
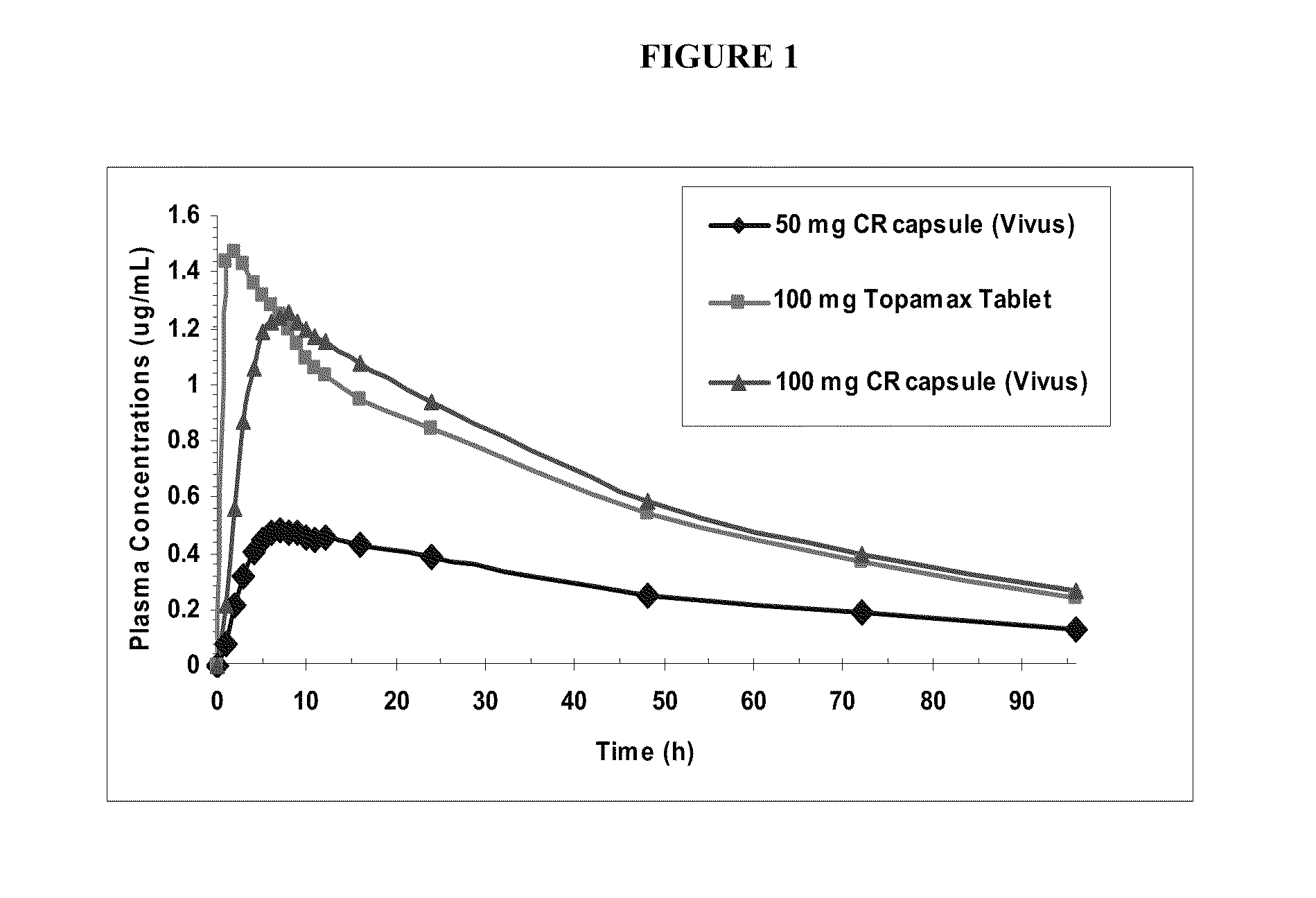
Taste masked topiramate composition and an orally disintegrating tablet comprising the same
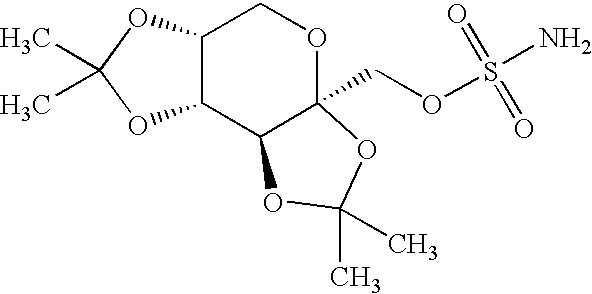
In various embodiments, the present invention is directed to a taste masked pharmaceutical composition comprising a therapeutically effective amount of taste masked sulfamate-substituted monosaccharide particles comprising a sulfamate-substituted monosaccharide or a pharmaceutically acceptable salt or derivative thereof that are coated with one or more taste-masking layers, and optionally one or more of taste-masked neltrexone, 5-HT3 receptor antagonist, phentermine, and vitamin B-12. The present invention relates to methods of making the taste masked and ODT compositions, and methods of using the compositions for treating a patient subject to an epileptic condition, migraines, dysphagia, achieving / maintaining weight loss, or alcoholism or drug addiction.
Owner:APTALIS PHARMATECH
Compositions and methods for treating obesity and related disorders
InactiveUS20080255093A1Reduces unwanted side effectGood for weight lossBiocideNervous disorderSYMPATHOMIMETIC AGENTSSerotonin receptor agonist
The present invention is drawn to combinations of pharmaceutical agents having similar chemical and / or pharmacological properties, wherein the combinations maximize the therapeutic effect of the drug while minimizing their adverse effects. The methods and compositions of the invention are particularly useful in the treatment of obesity and related conditions which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) or bupropion in combination with an anti-epileptic agent (e.g., topiramate, zonisamide), CB1 antagonists (e.g., rimonabant), or a 5HT2C-selective serotonin receptor agonist, (e.g., lorcaserin) for the treatment of obesity and related conditions. The invention also features kits for use in the practice of these novel therapies.
Owner:VIVUS
Combination Therapy
The present invention features a novel therapy for treating diabetes, hypertension, migraine, epilepsy, sleep apnea, depression, impulse control disorders or alcohol addiction which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate compound (e.g., topiramate) or an anticonvulsive sulfonylurea compound (e.g. zonisamide).
Owner:VIVUS
Combination of an H3 antagonist/inverse agonist and an appetite suppressant
The present invention relates to pharmaceutical compositions comprising therapeutic combinations comprising: one or more H3 antagonists / inverse agonists; one or more appetite suppressants selected from the group consisting of CB1 antagonists / inverse agonists, sibutramine, phentermine and topiramate; and optionally one or more HMG-CoA reductase inhibitors. The invention also relates to medicaments and kits comprising the pharmaceutical compositions of the present invention, and methods of treating obesity, obesity related disorders and diabetes using the pharmaceutical compositions of the present invention.
Owner:SCHERING CORP
Escalating dosing regimen for effecting weight loss and treating obesity
The present invention is drawn to novel topiramate compositions as well as methods for effecting weight loss, e.g., in the treatment of obesity and related conditions, including conditions associated with and / or caused by obesity per se. The present invention features an escalating dosing regimen adapted for the administration of topiramate and optionally a sympathomimetic agent such as phentermine or bupropion, in the treatment of obesity and related conditions.
Owner:VIVUS LLC
Combination therapy for effecting weight loss and treating obesity
InactiveUS7674776B2Improve side effectsAmeliorating sleep apneaBiocideMetabolism disorderSYMPATHOMIMETIC AGENTSCombined Modality Therapy
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Low dose topiramate / phentermine composition and methods of use thereof
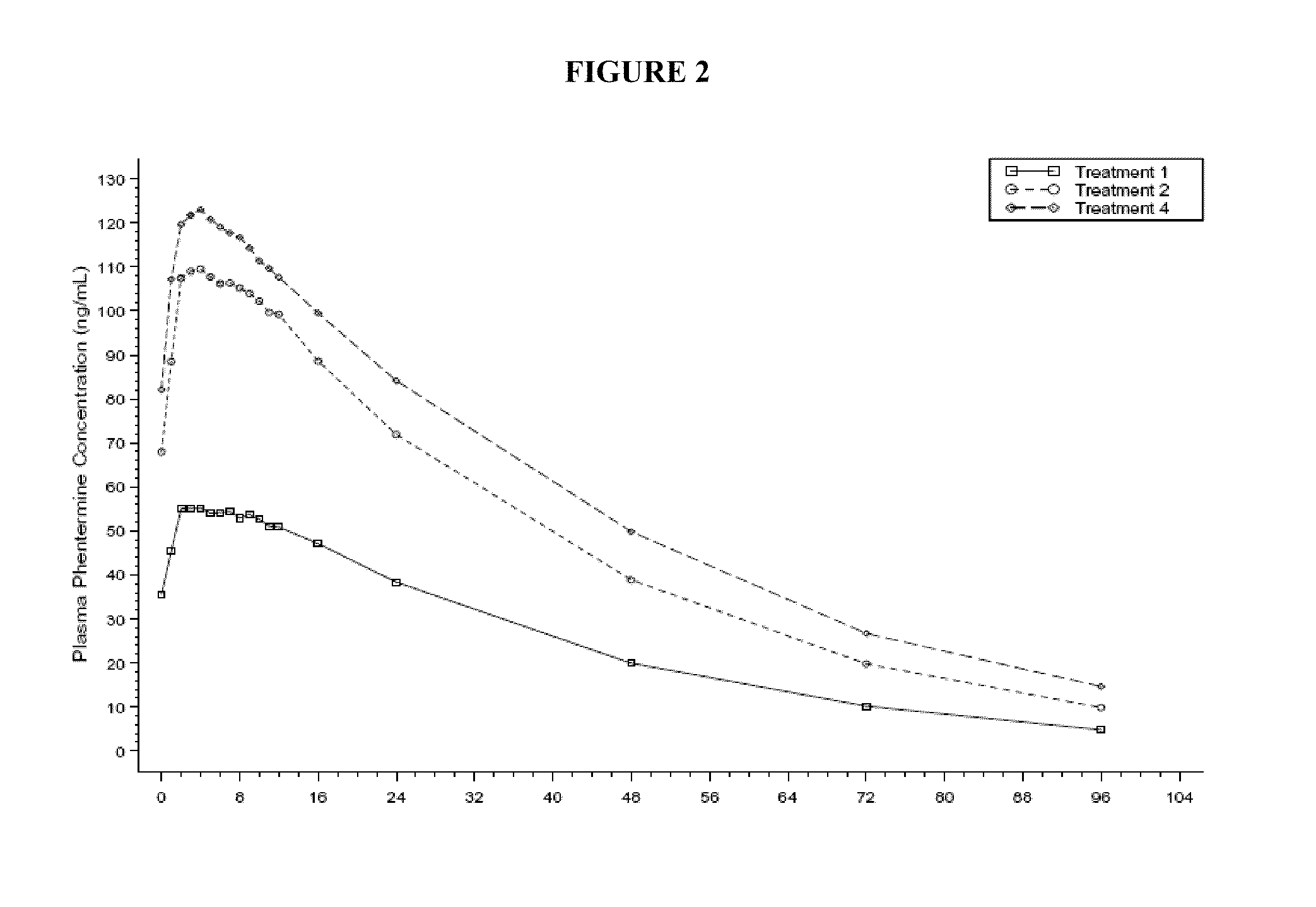
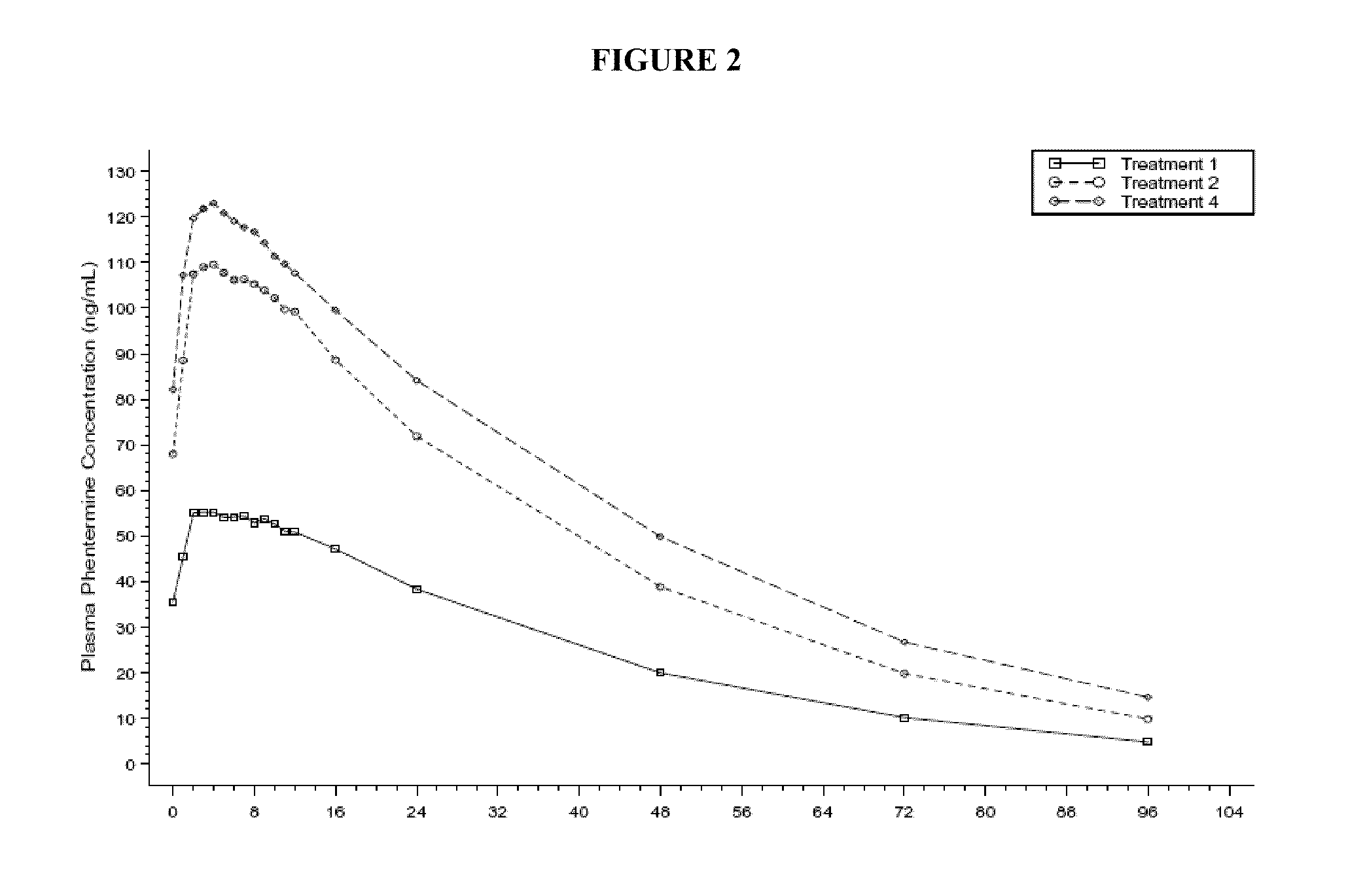
A method for effecting weight loss by administering a combination of topiramate and phentermine is provided. The phentermine is generally administered in immediate release form, in a daily dose in the range of 2 mg to 8 mg, in combination with a daily dose of topiramate selected to prevent the loss of effectiveness of phentermine alone. Methods for treating obesity, conditions associated with obesity, and other indications are also provided, as are compositions and dosage forms containing low doses of phentermine and topiramate, e.g., 3.75 mg phentermine and 23 mg topiramate.
Owner:VIVUS LLC
Combination Therapies for the Treatment of Obesity
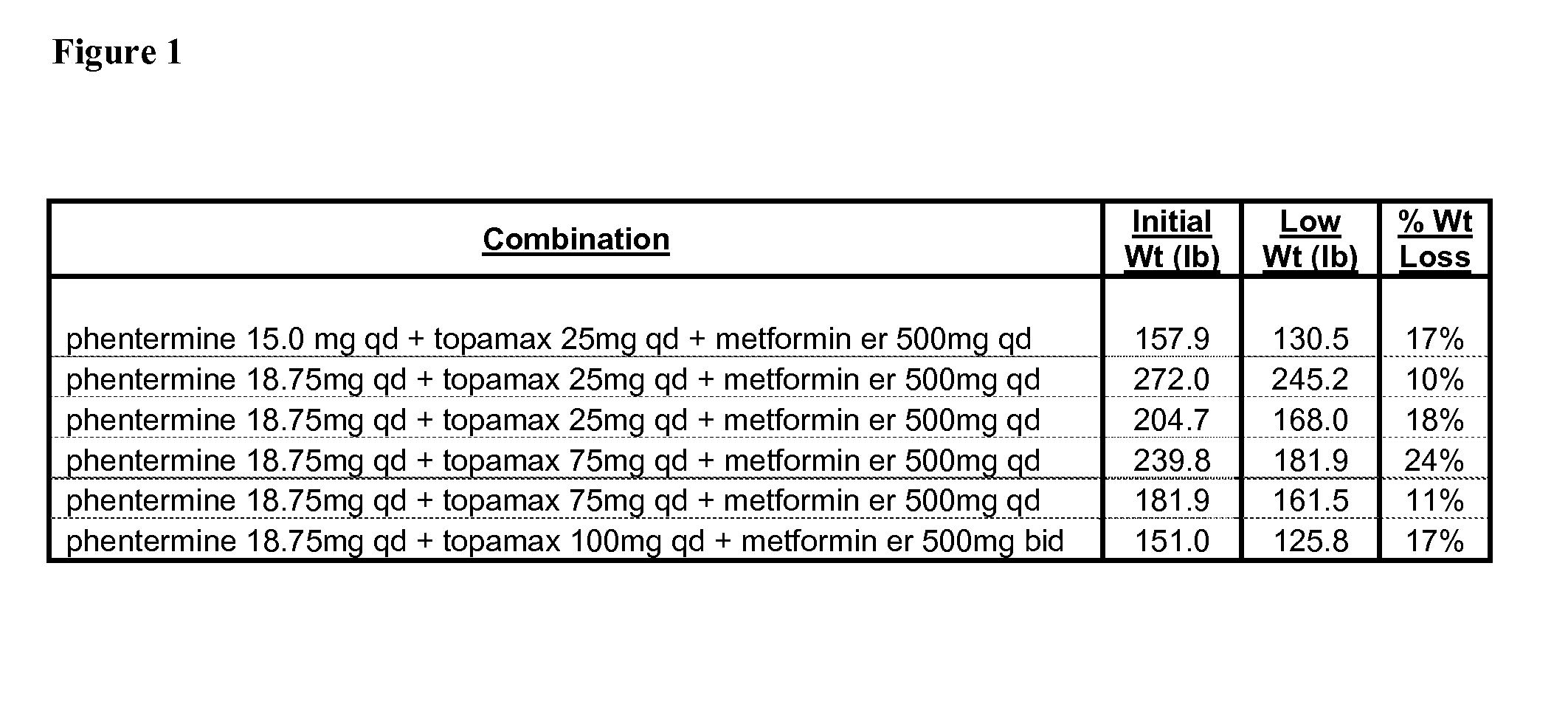
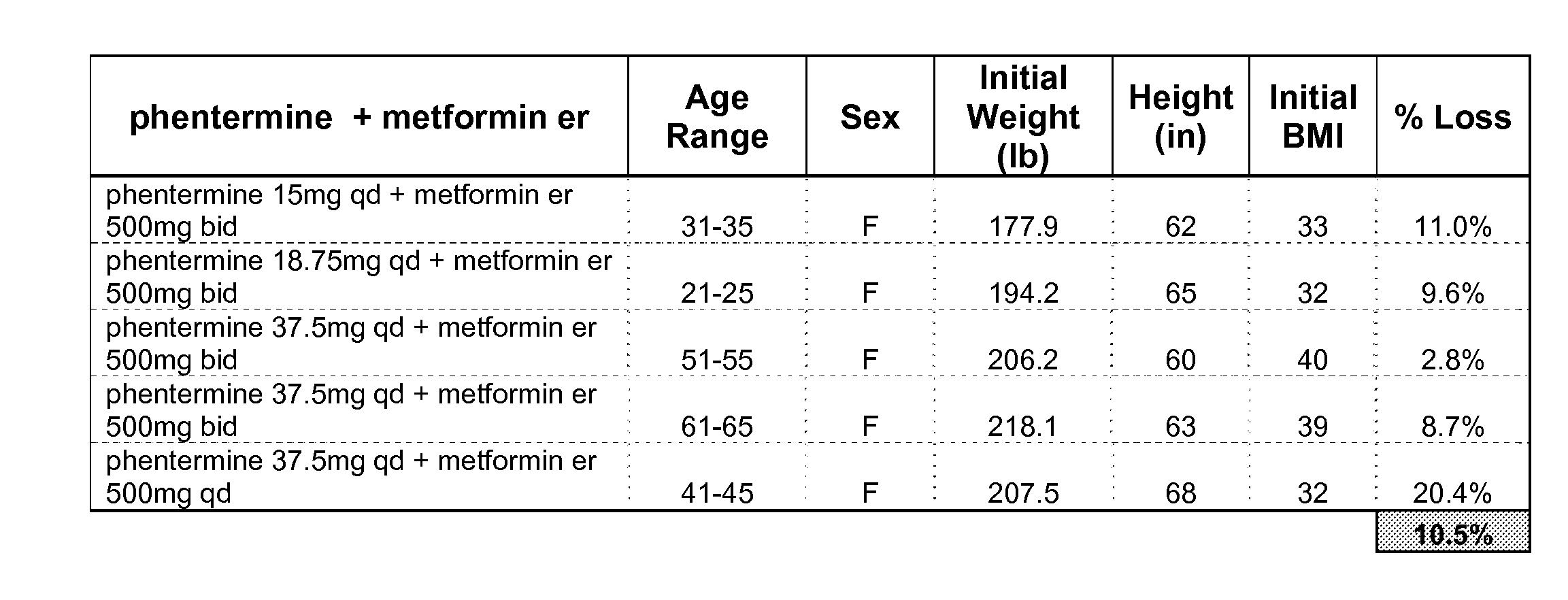
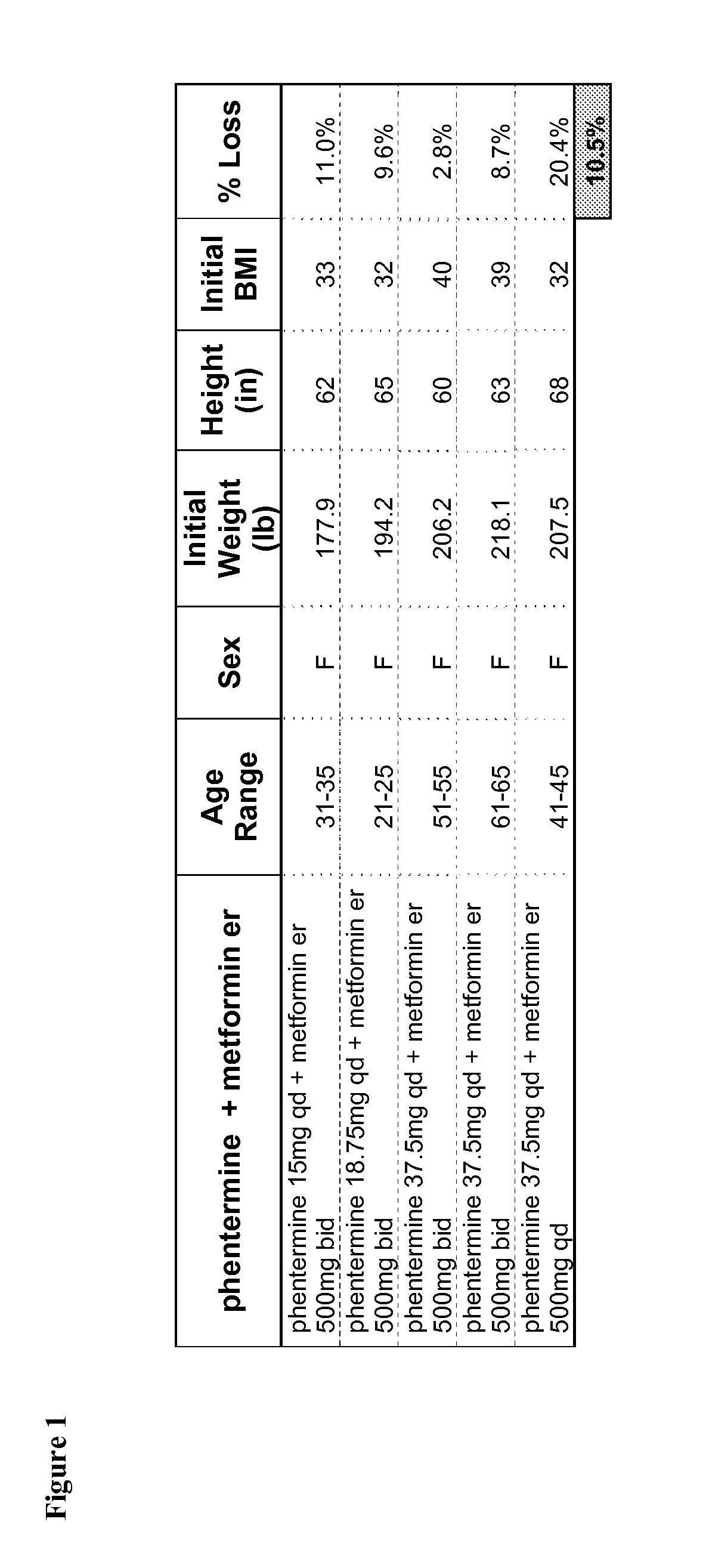
Described are pharmaceutical compositions comprising topiramate, phentermine, and metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of topiramate, phentermine, and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Comprehensive pharmacologic therapy for treatment of obesity including cysteine
InactiveUS20050065190A1Simple and inexpensive designWithout fear of injury to personsBiocidePeptide/protein ingredientsDiethylpropionVitamin C
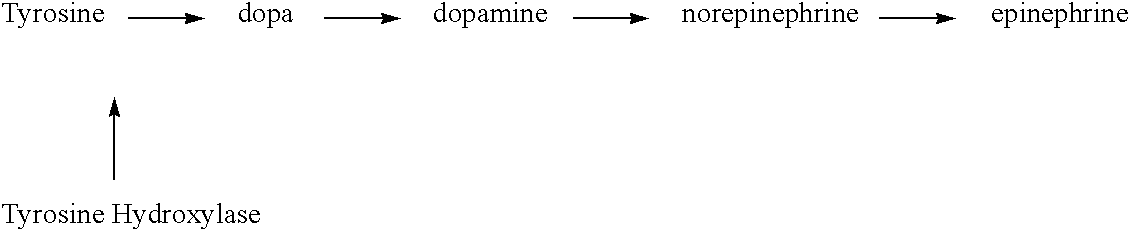
The comprehensive pharmacologic therapy for treatment of obesity including Cysteine is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, Cysteine, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
Combination therapies for the treatment of obesity
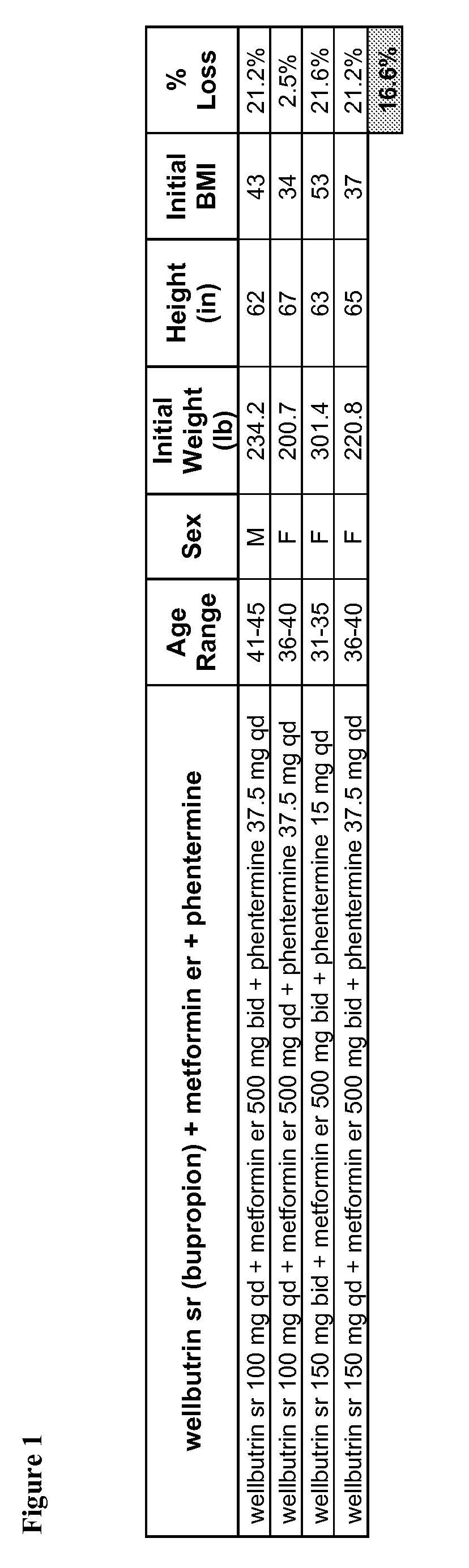
Described are pharmaceutical compositions comprising bupropion, metformin, phentermine, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of bupropion, metformin, and phentermine. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Combination therapy for effecting weight loss and treating obesity
InactiveUS7553818B2Improve side effectsAmeliorating sleep apneaBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSCombined Modality Therapy
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss.The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Low Dose Topiramate / Phentermine Composition and Methods of Use Thereof
A method for effecting weight loss by administering a combination of topiramate and phentermine is provided. The phentermine is generally administered in immediate release form, in a daily dose in the range of 2 mg to 8 mg, in combination with a daily dose of topiramate selected to prevent the loss of effectiveness of phentermine alone. Methods for treating obesity, conditions associated with obesity, and other indications are also provided, as are compositions and dosage forms containing low doses of phentermine and topiramate, e.g., 3.75 mg phentermine and 23 mg topiramate.
Owner:VIVUS
Combination therapy for effecting weight loss and treating obesity
InactiveUS7659256B2Improve side effectsAmeliorating sleep apneaBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSSympatholytic Drugs
Owner:VIVUS
Combination therapy for effecting weight loss and treating obesity
InactiveUS20060234952A1Ameliorating sleep apneaLowering blood blood glucose blood levelBiocideMetabolism disorderSYMPATHOMIMETIC AGENTSPhentermine
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss. The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
Escalating dosing regimen for effecting weight loss and treating obesity
The present invention is drawn to novel topiramate compositions as well as methods for effecting weight loss, e.g., in the treatment of obesity and related conditions, including conditions associated with and / or caused by obesity per se. The present invention features an escalating dosing regimen adapted for the administration of topiramate and optionally a sympathomimetic agent such as phentermine or bupropion, in the treatment of obesity and related conditions.
Owner:VIVUS LLC
Combination therapy for effecting weight loss and treating obesity
InactiveUS20060234950A1Ameliorating sleep apneaLowering blood blood glucose blood levelBiocideCarbohydrate active ingredientsSYMPATHOMIMETIC AGENTSPhentermine
The present invention features a novel therapy for effecting weight loss which involves treating a subject with a sympathomimetic agent (e.g., phentermine or a phentermine-like drug) in combination with an anticonvulsant sulfamate derivative (e.g., topiramate) such that the subject experiences weight loss. The combination methods of the present invention also are effective against symptoms associated with Syndrome X. The invention also features pharmaceutical compositions and kits for use in the practice of these novel therapies.
Owner:VIVUS
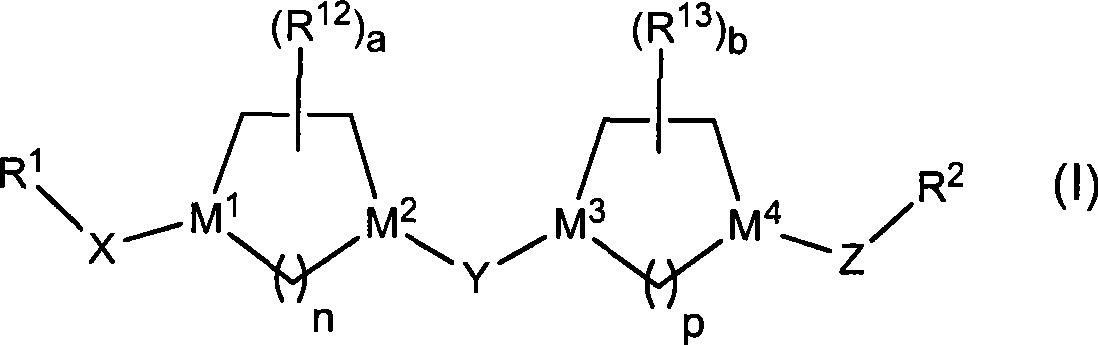
5ht2C receptor modulator compositions
InactiveUS8153621B2Decreasing food intakeDecreasing food intake of a mammalBiocideMetabolism disorderPhentermineObesity
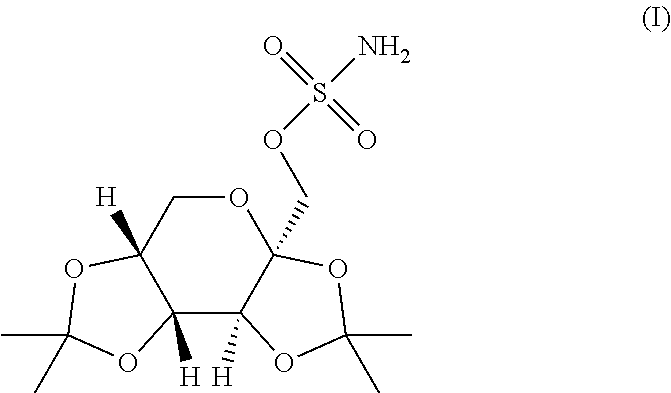
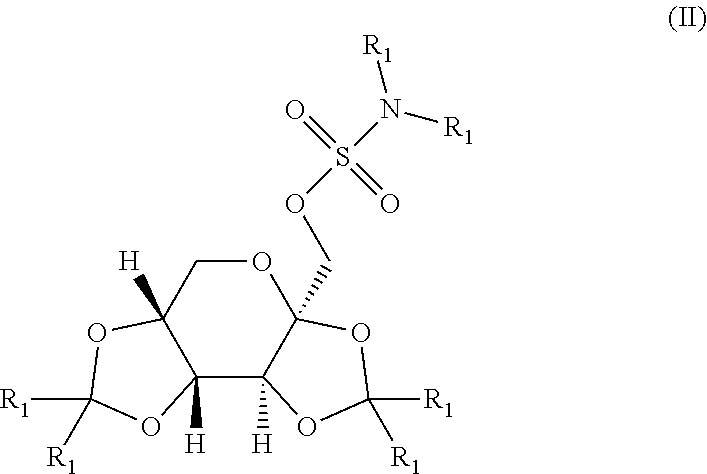
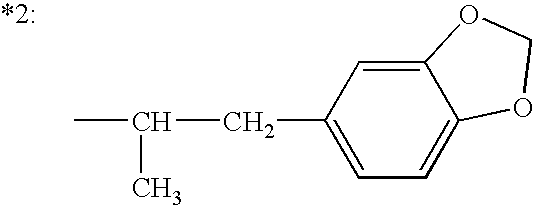
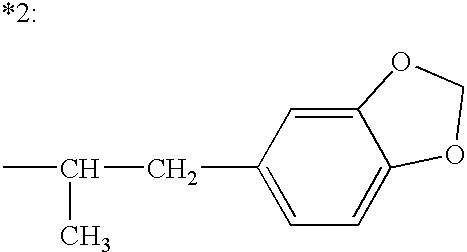
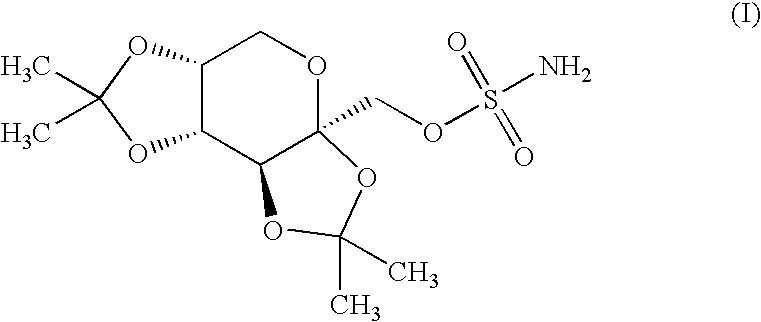
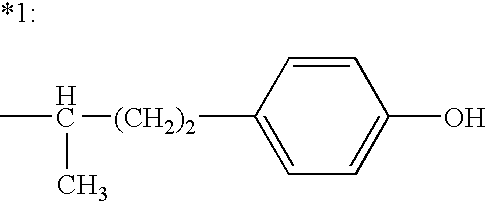
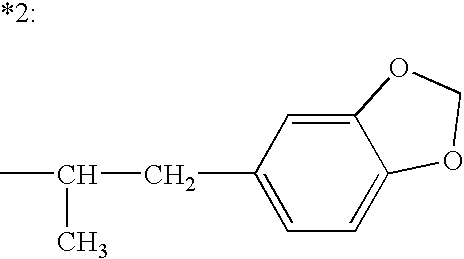
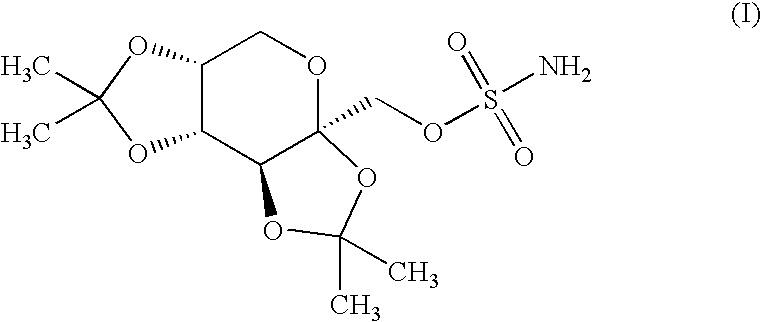
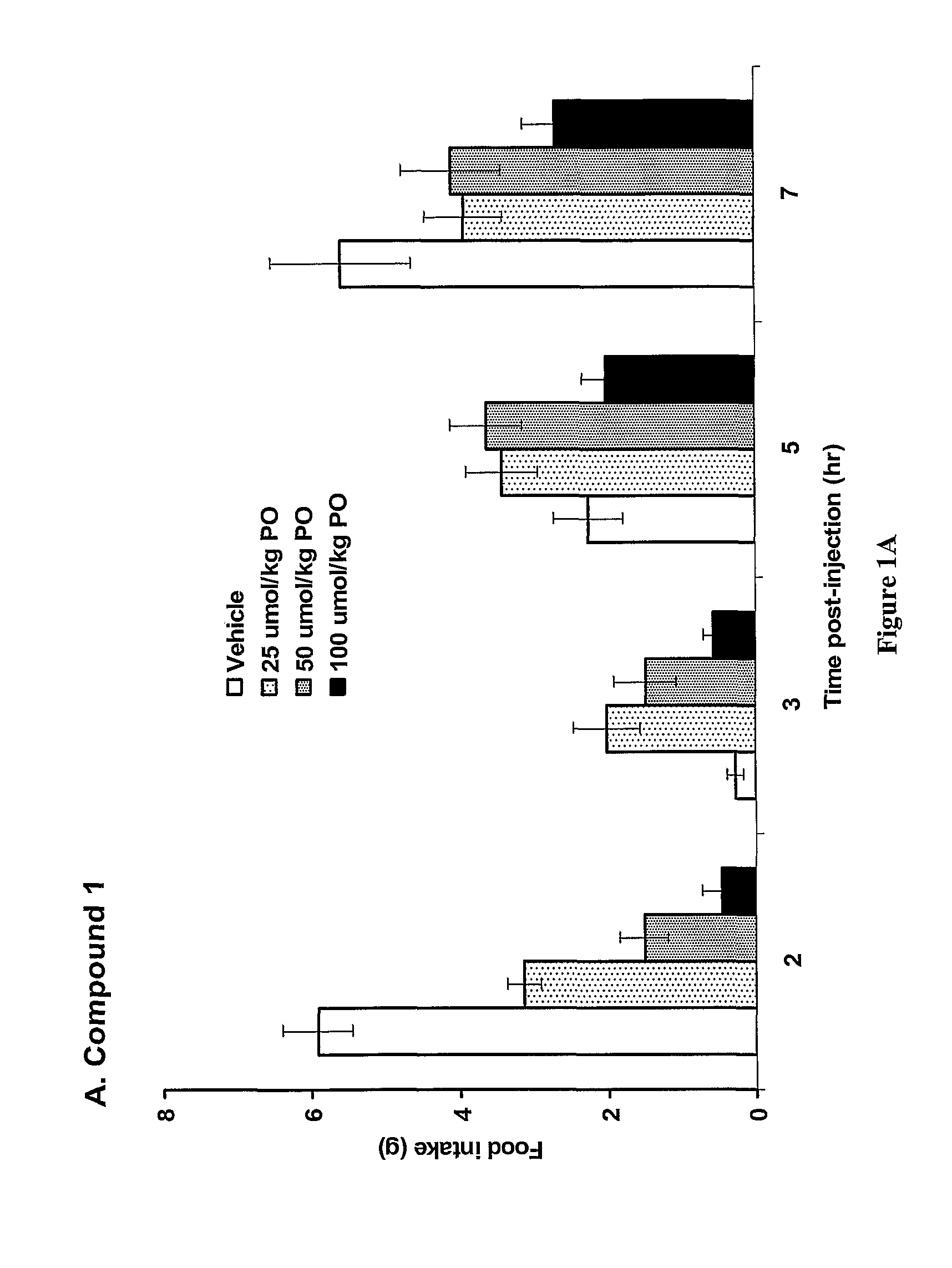
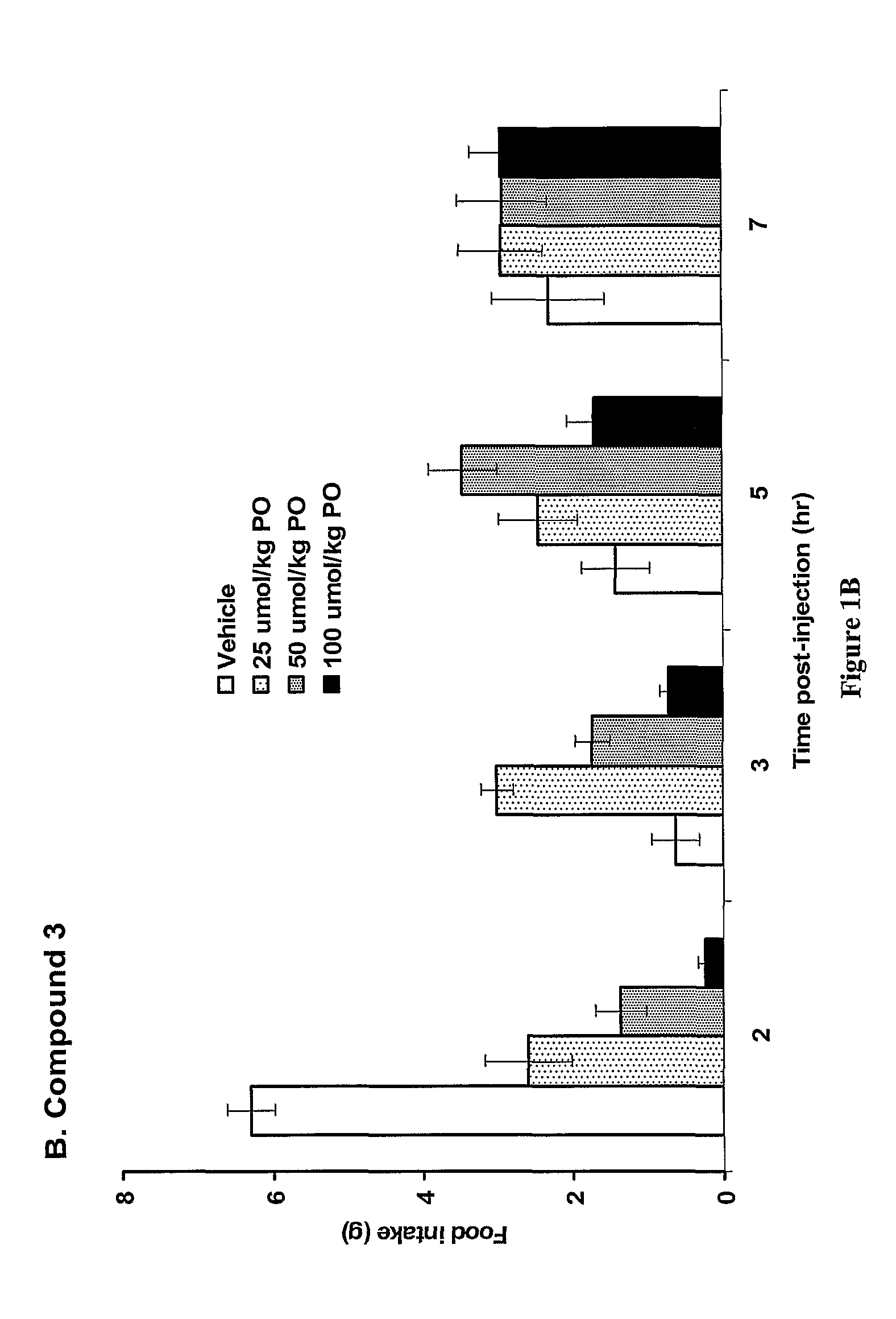
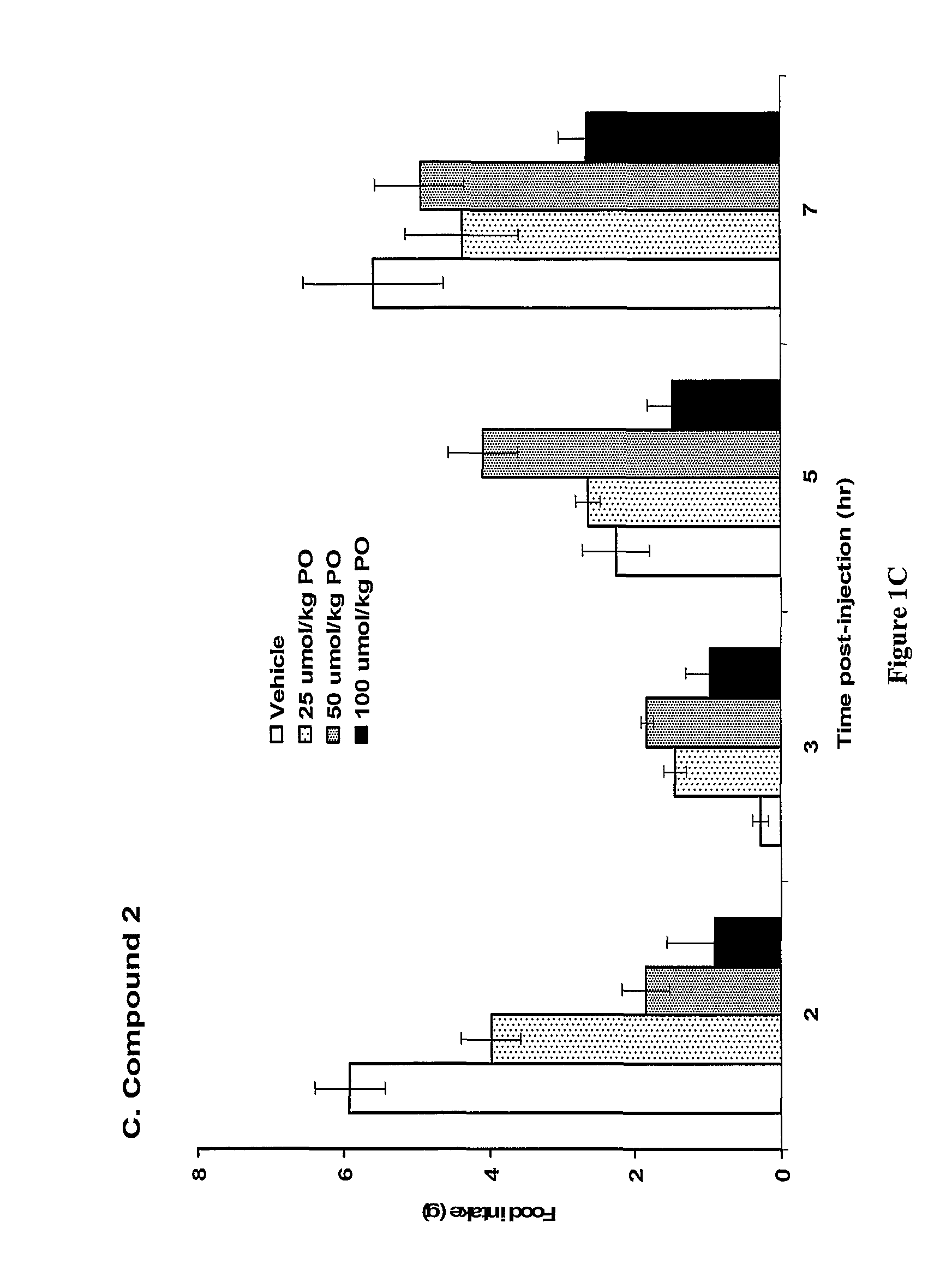
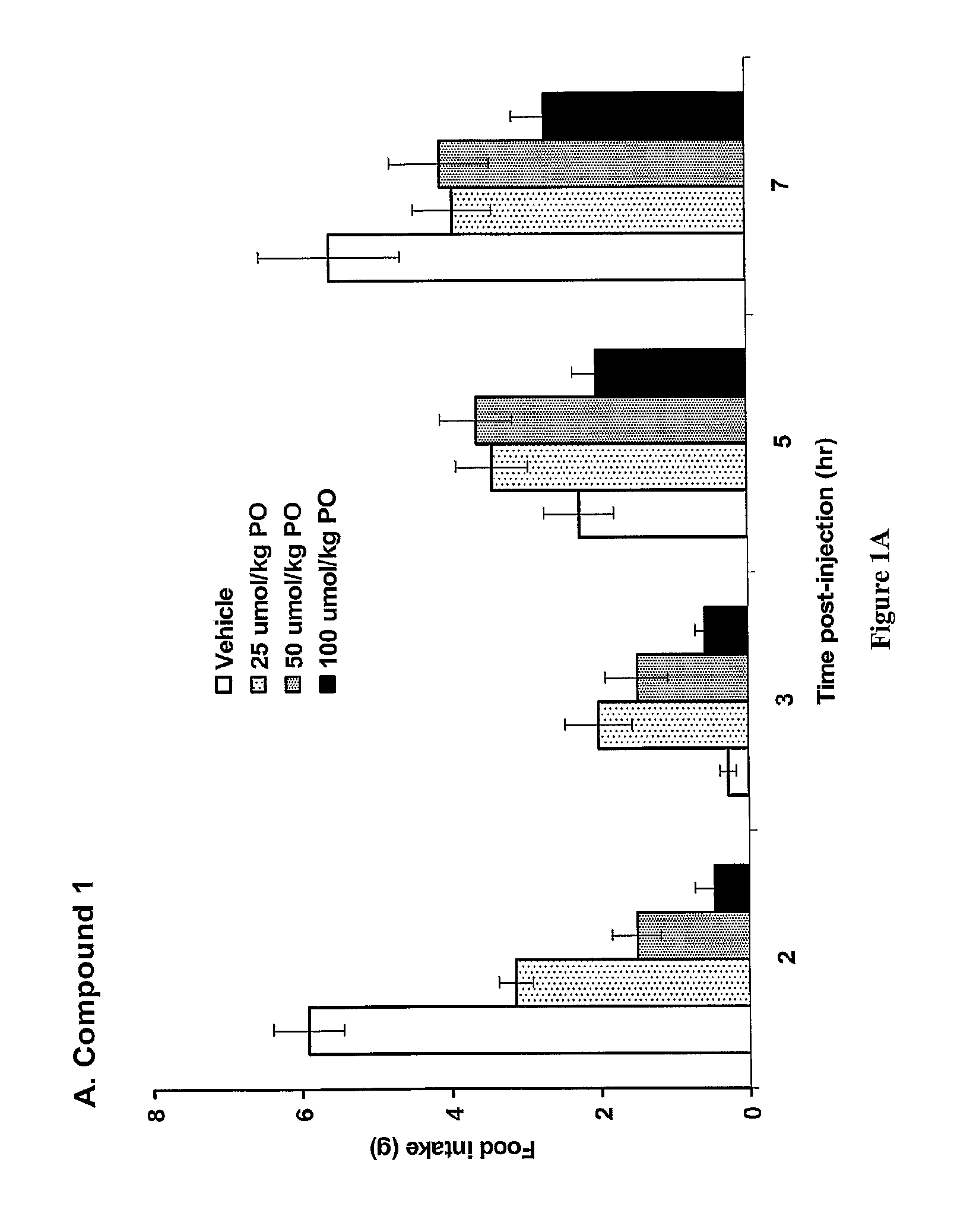
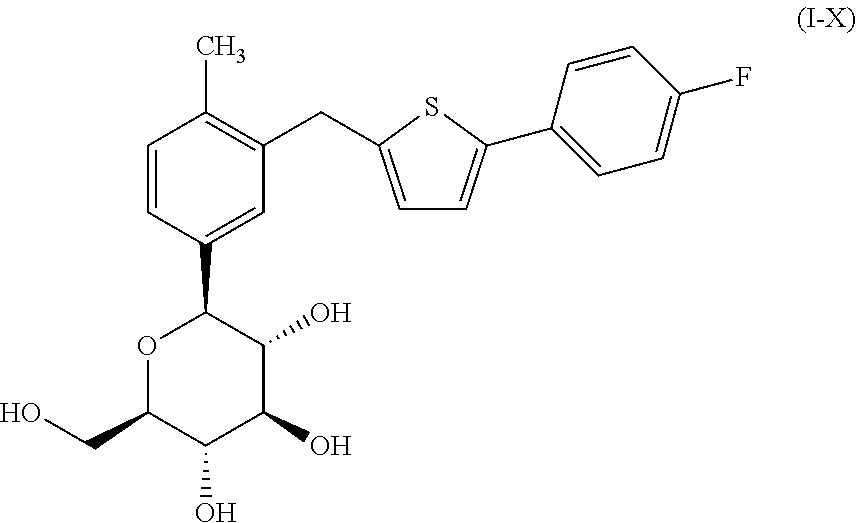
The present invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist. In addition, the invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist having Formula (I):or a pharmaceutically acceptable salt, solvate or hydrate thereof. These compositions are useful in pharmaceutical compositions whose use includes the treatment of obesity.
Owner:ARENA PHARMA
Comprehensive pharmacologic therapy for treatment of a dysfunction
InactiveUS20050233008A1Promote resultsHigh expectation of weight lossBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
5ht2c receptor modulator compositions and methods of use
InactiveUS20120252786A1Decreasing food intakeDecreasing food intake of a mammalBiocideMetabolism disorderPhentermineObesity
The present invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist. In addition, the invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist having Formula (I): or a pharmaceutically acceptable salt, solvate or hydrate thereof. These compositions are useful in pharmaceutical compositions whose use includes the treatment of obesity.
Owner:ARENA PHARMA
Treatment of obstructive sleep apnea syndrome with a combination of a carbonic anhydrase inhibitor and an additional active agent
InactiveUS20110224196A1Amelioration of the extent of each apneaAlleviate excessive daytime sleepinessBiocideNervous disorderZaleplonActive agent
This invention relates generally to methods and pharmaceutical formulations useful in treating patients suffering from obstructive sleep apnea syndrome (OSAS). Treatment of OSAS is effected by administering a carbonic anhydrase inhibitor to the patient in combination with at least one additional active agent. Examples of additional active agents include modafinil, eszopiclone, zolpidem, zaleplon, and phentermine.
Owner:VIVUS
Comprehensive pharmacologic therapy for treatment of obesity including cysteine
InactiveUS7268161B2Simple and inexpensive designEffective therapyBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity including Cysteine is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, Cysteine, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:FEDERAL LAW ENFORCEMENT DEV SERVICES
Combination Therapies for the Treatment of Obesity
InactiveUS20100331999A1Reduce weightOrganic active ingredientsBiocideCombined Modality TherapyPhentermine
Described are pharmaceutical compositions comprising phentermine, metformin, and at least one pharmaceutically acceptable carrier or excipient. Another aspect of the present invention relates to a method of treating a patient suffering from obesity or needing to lose weight, comprising the step of co-administering to said patient a therapeutically effective amount of phentermine and metformin. In certain embodiments, an aforementioned method is practiced in conjunction or tandem with a medical procedure or the use of a medical device or both.
Owner:METABOLOUS PHARMA
Comprehensive pharmacologic therapy for treatment of obesity
InactiveUS20060135567A1High expectation of weight lossPromote resultsBiocidePeptide/protein ingredientsDiethylpropionVitamin C
The comprehensive pharmacologic therapy for treatment of obesity is a procedure which involves the administration of a desired therapeutic range of Diethylpropion and / or Phentermine in combination with a SSRI medication and nutritional supplementation for brief and long durations which may be 12 months or more. The preferred procedure involves the administration of drugs in combination which are identified as: Citalopram (Celexa) and Phentermine; Citalopram (Celexa) and Diethylpropion; Citalopram (Celexa), Phentermine, and Diethylpropion. In addition nutritional supplementation such as a multivitamin, 5-Hydroxytryptophan, vitamin B6, vitamin C, Tyrosine, Calcium, and Lysine may be used to enhance the performance of the weight loss treatment program.
Owner:HINZ MARTIN C
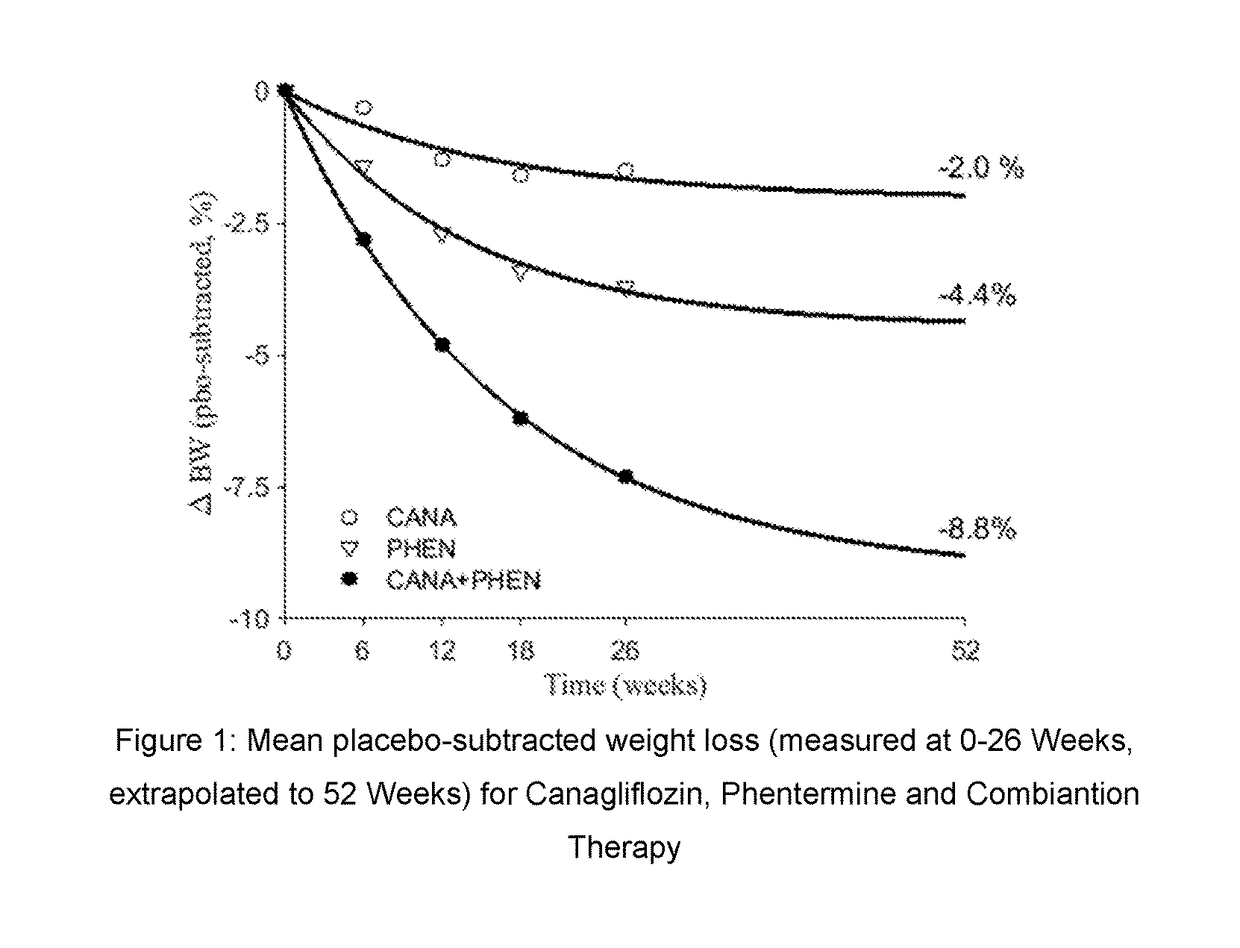
Co-therapy comprising canagliflozin and phentermine for the treatment of obesity and obesity related disorders
InactiveUS20170071970A1Inhibit oxidative stressAltered expressionOrganic active ingredientsMetabolism disorderPhentermineAdjuvant therapy
The present invention is directed to the use of co-therapy comprising administration of canagliflozin and phentermine for the treatment of obesity and obesity related disorders. More particularly, the present invention is directed to co-therapy for treating obesity, for promoting weight loss and / or for suppressing appetite; for treating, delaying, slowing the progression of and / or preventing metabolic disorders (including for example Type 2 diabetes mellitus); for treating, delaying, slowing the progression of and / or preventing renal or fatty liver disorders (including for example NASH, NAFLD, etc.); for treating, delaying, slowing the progression of and / or preventing sleep disorders (including for example sleep apnea); for providing cardiovascular protection; for treating, delaying, slowing the progression of and / or preventing cardiovascular events (including major adverse cardiac events (MACE) such as myocardial infarction, unstable angina, cardiovascular death, revascularization, fatal or non-fatal cerebrovascular accident, peripheral arteriopathy, aortic events, hospitalization due to congestive heart failure, etc.); and / or for extending or prolonging life span.
Owner:JANSSEN PHARMA NV
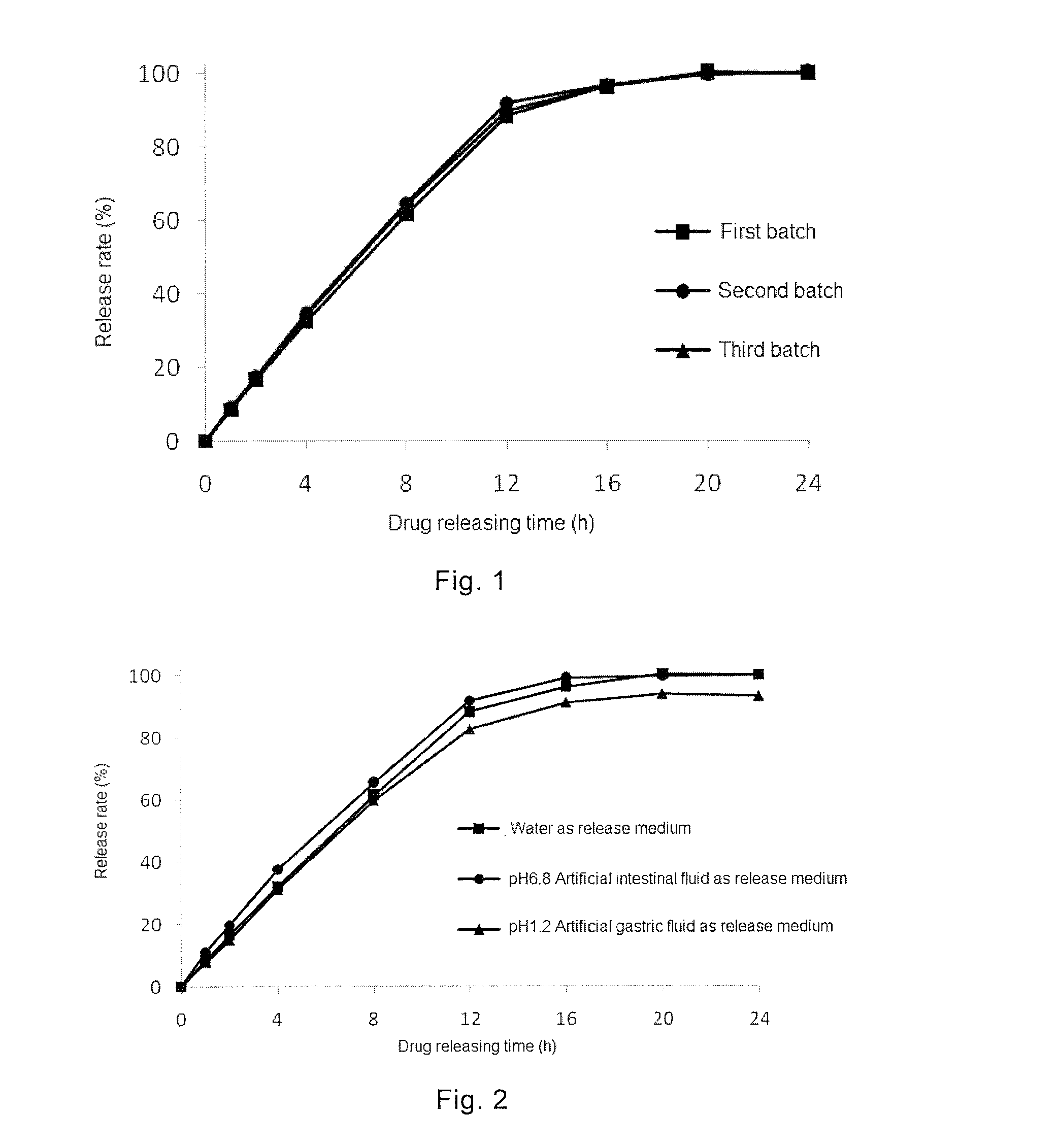
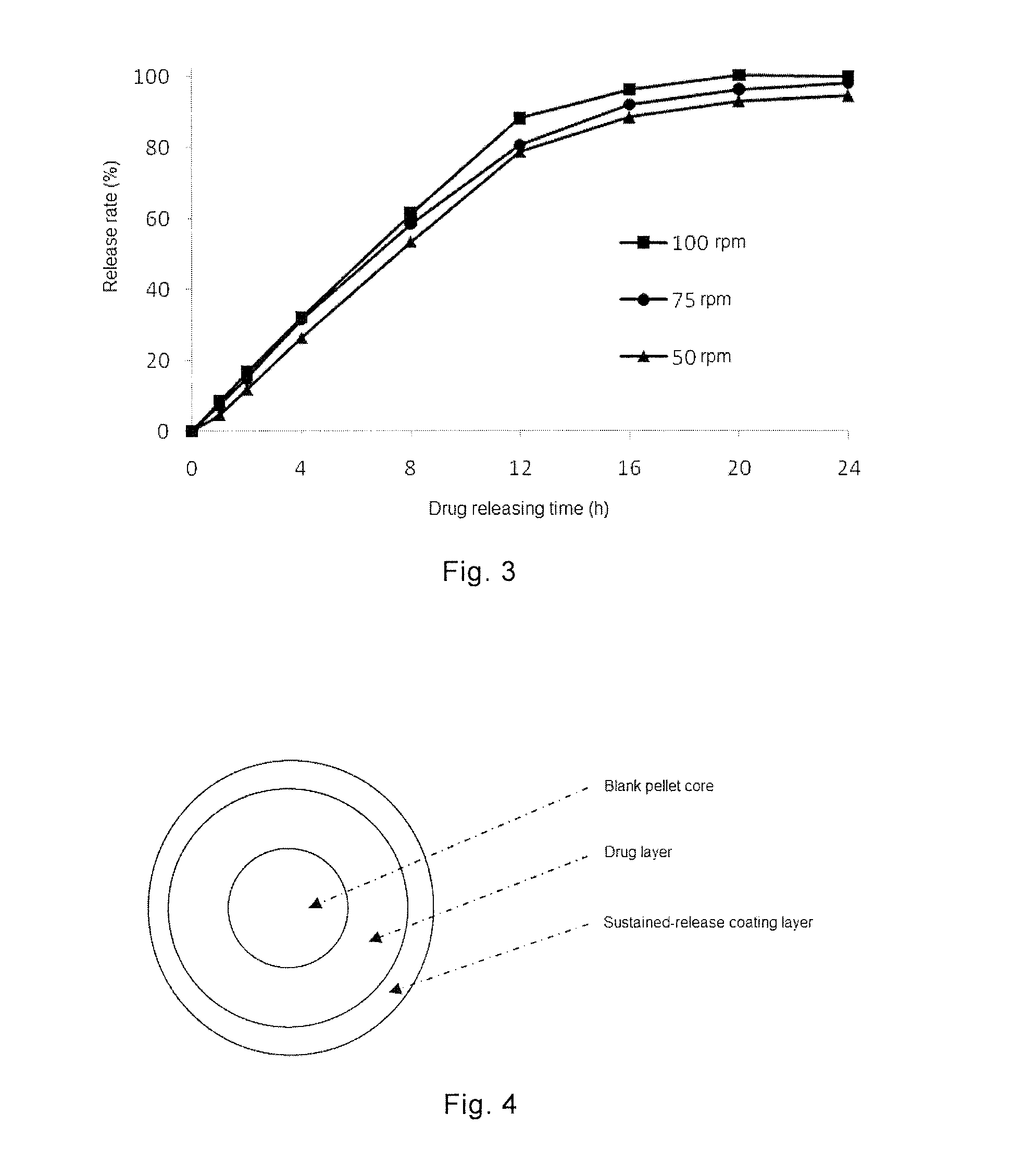
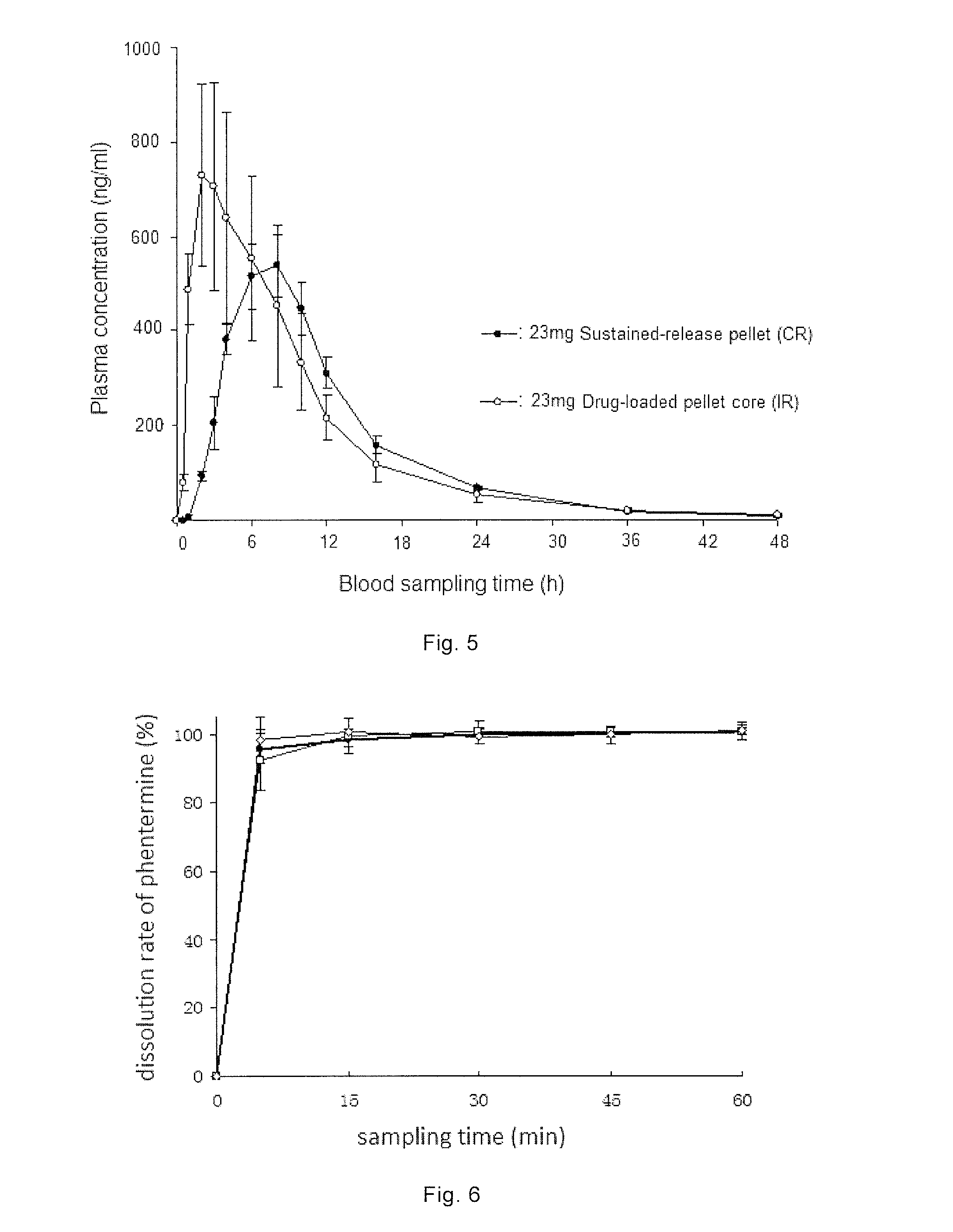
Combination Product Comprising Phentermine and Topiramate, and Preparation Method Thereof
ActiveUS20150044295A1Simple manufacturing methodDesired drug releaseBiocideNervous disorderSustained release pelletsImmediate release
The present invention provides a combination product, which comprises immediate release pellet of phentermine and sustained-release pellet of topiramate, wherein the pellet of topiramate includes: a) a blank pellet core; b) an active drug layer that contains topiramate and is free of binding agent, the layer being located on surface of the blank pellet core; c) a sustained-release coating layer containing ethyl cellulose and PVP K30, the sustained-release coating layer being located on external of the active drug layer. The present invention further discloses a method for preparing the combination product.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Combination of an h3 antagonist/inverse agonist and an appetite suppressant
The present invention relates to pharmaceutical compositions comprising therapeutic combinations comprising: one or more H3 antagonists / inverse agonists; one or more appetite suppressants selected from the group consisting of CBi antagonists / inverse agonists, sibutramine, phentermine and topiramate; and option one or more HMG-CoA reductase inhibitors. The invention also relates to medicaments and kits comprising the pharmaceutical compositions of the present invention, and methods of treating obesity, obesity related disorders and diabetes using the pharmaceutical compositions of the present invention.
Owner:SCHERING AG
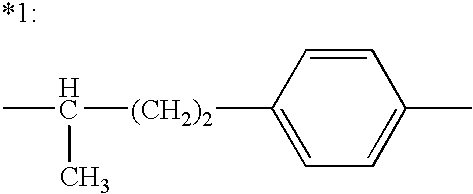
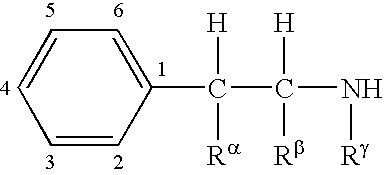
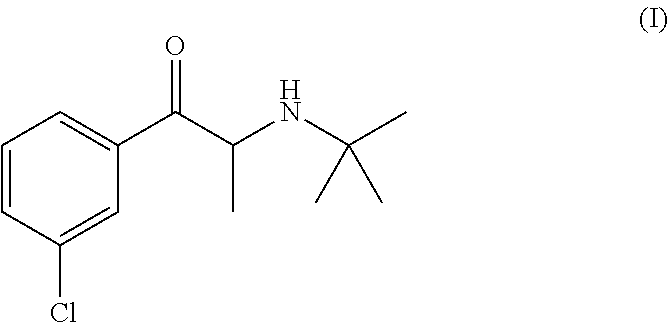
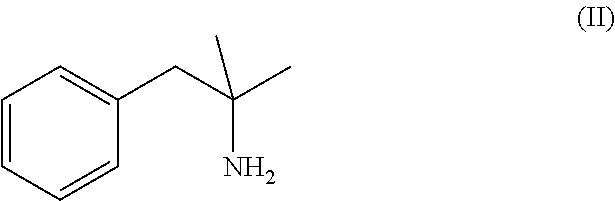
Methods for treating attention deficit hyperactivity disorder using a combination of bupropion ((±)-2-(tert-butylamino)-1-(3-chlorophenyl)propan-1-one) and phentermine (2-methyl-1-phenylpropan-2-amine)
Compositions and methods for treating attention deficit hyperactivity disorder are disclosed. Compositions for treating attention deficit hyperactivity disorder include bupropion, phentermine and a psychostimulant drug. Methods for treating attention deficit hyperactivity disorder include administering to an individual in need a composition including bupropion and phentermine Methods for treating attention deficit hyperactivity disorder can also include further administering to an individual in need a psychostimulant drug.
Owner:WINSTON THOMAS R
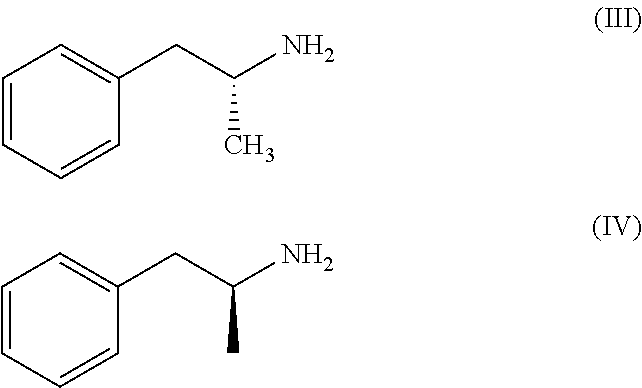
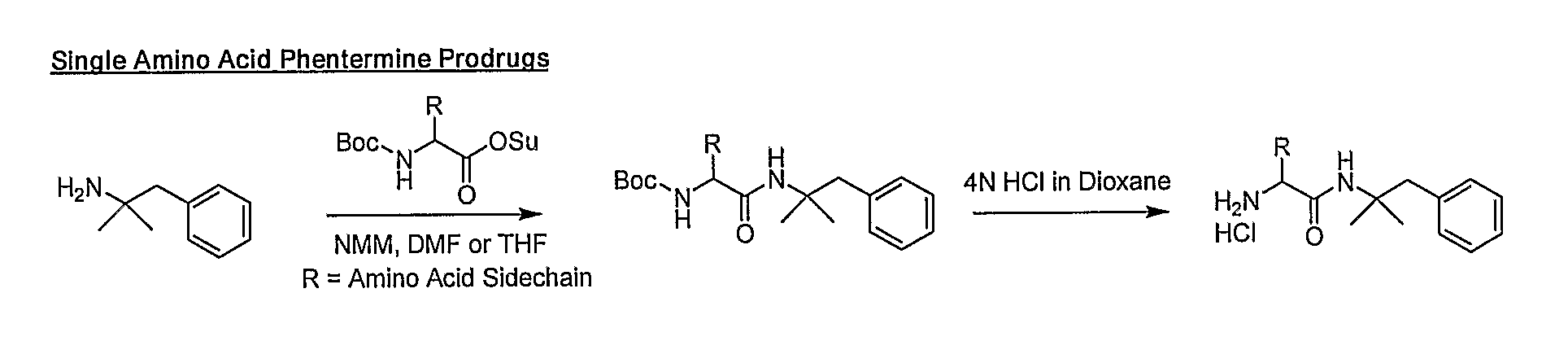
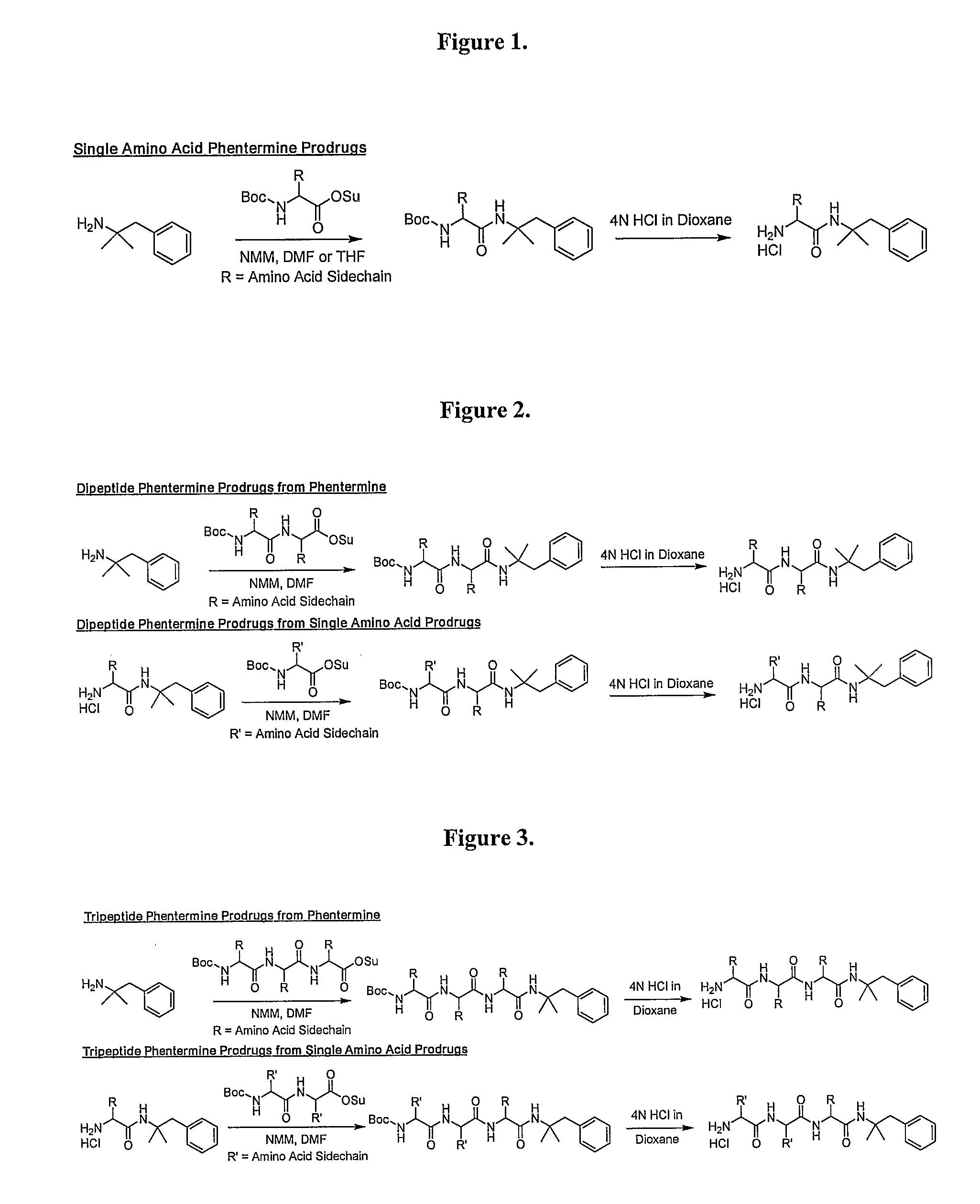
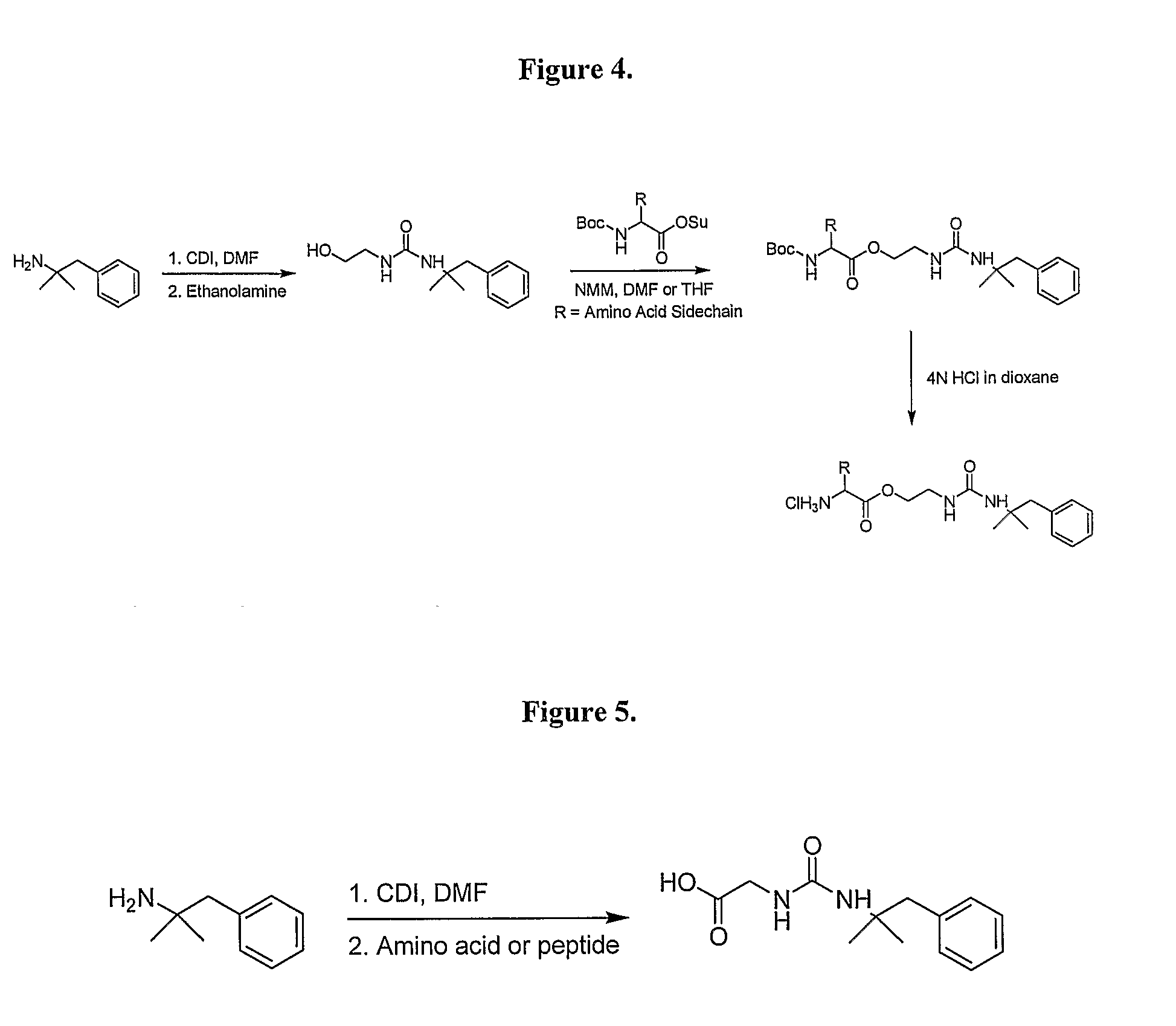
Prodrugs of Phentermine
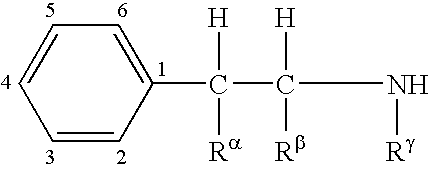
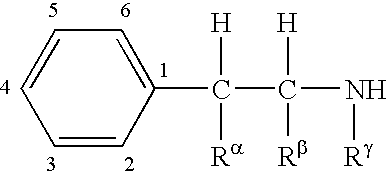
The invention relates to compositions of amino acid and peptide conjugates comprising phentermine. Phentermine is covalently attached to at least one amino acid via its amine group to the N-terminus, the C-terminus, a side chain of the peptide carrier. Also discussed are methods for treating obesity.
Owner:SHIRE PLC
5ht2c receptor modulator compositions and methods of use
InactiveUS20090197868A1Control weight gainDecreasing food intakeBiocideMetabolism disorderAgonistPhentermine
The present invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist. In addition, the invention relates to a composition comprising phentermine and a selective 5HT-2C receptor agonist having Formula (I): or a pharmaceutically acceptable salt, solvate or hydrate thereof. These compositions are useful in pharmaceutical compositions whose use includes the treatment of obesity.
Owner:ARENA PHARMA
Weight-losing food and preparation method thereof
InactiveCN109329928AReduce dependenceEasy to operateNatural extract food ingredientsFood ingredient as mouthfeel improving agentSide effectInulin
The present invention discloses weight-losing food. The weight-losing food is prepared from the following raw materials in parts by weight: 50-60 parts of phentermine modified inulin, 5-10 parts of milk, 1-3 parts of grapefruit peel, 1-2 parts of cream, 5-10 parts of a traditional Chinese medicinal composition extract, 1-3 parts of baking powder and 15-25 parts of water. The present invention alsodiscloses a preparation method of the weight-losing food. The disclosed weight-losing food has a good curative effect on treating obesity, is free of side effects and can also provide comprehensive and balanced nutrients for the human body.
Owner:湖南博隽生物医药有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com