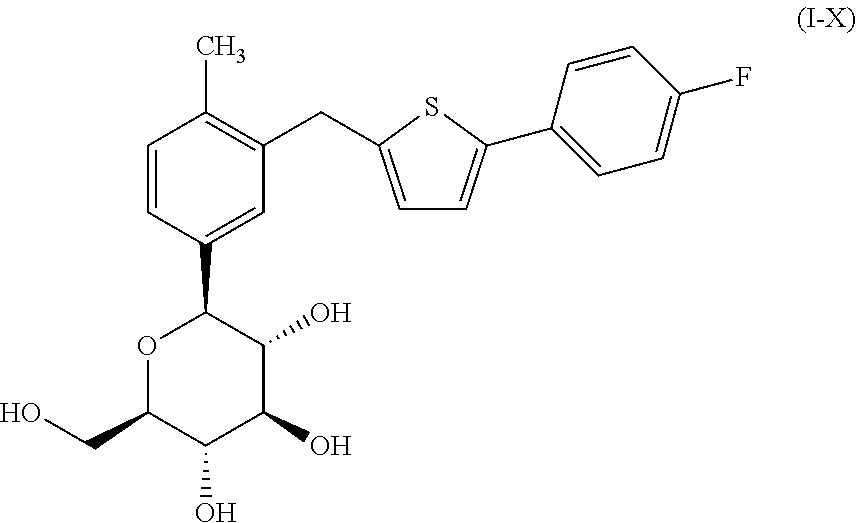
Co-therapy comprising canagliflozin and phentermine for the treatment of obesity and obesity related disorders
a technology which is applied in the field of co-therapy comprising the administration of canagliflozin and phentermine for the treatment of obesity and obesity related disorders, can solve the problems of increased risk of cardiovascular disease, so as to reduce the risk of cardiovascular disease and improve the effect of cardiovascular diseas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Trial
Co-Therapy with 300 mg Canagliflozin and 15 mg Phentermine
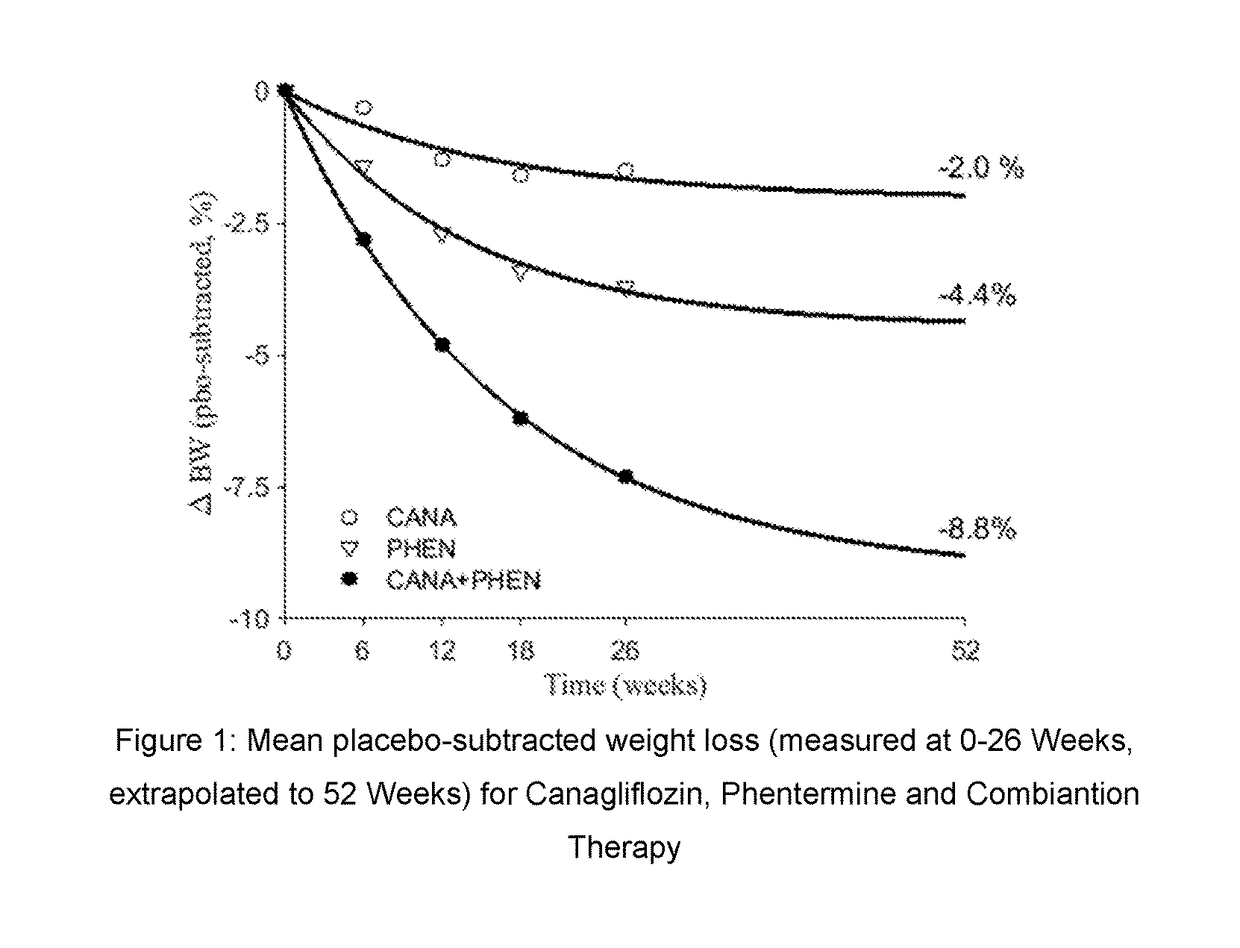
[0281]The safety and efficacy of combination treatment with 300 mg canagliflozin and 15 mg phentermine was investigated in a 26 week, randomized, double-blind, placebo-controlled, parallel group, multi-center study. (Complete study protocol filed and available as STUDY 28431754-OBE2002 on www.clinicaltrials.gov).
Trial Design:
[0282]The study began with a 4-week single blind placebo run-in period. After completing the run-in period 335 overweight or obese non-diabetic adult subjects who had a BMI≧230 kg / m2 and 2 at screening; or BMI≧227 kg / m2 and 2 at screening in the presence of a comorbidity / comorbidities of hypertension and / or dyslipidemia were randomly assigned in a 1:1:1:1 ratio to treatment with (A) canagliflozin 300 mg and phentermine 15 mg, (B) canagliflozin 300 mg, (C) phentermine 15 mg, or (D) placebo with the stratification factor run-in weight loss of ≦2 kg or >2 kg. All subjects were provided with diet and exe...
formulation example 1
[0293]As a specific embodiment of an oral composition, 300 mg of canagliflozin and 15 mg of phentermine are formulated with sufficient finely divided lactose to provide a total amount of 580 to 590 mg to fill a size O hard gel capsule.
formulation example 2
[0294]As a specific embodiment of an oral composition, 300 mg of canagliflozin and 15 mg of phentermine are formulated with lactose and microcrystalline cellulose to provide a tablet of total weight in the amount of about 600 mg to about 620 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com