Metformin hydrochloride slowly released tablet and its preparation method
A technology of metformin hydrochloride and a preparation process, which is applied in metabolic diseases, pharmaceutical formulations, drug combinations and other directions, can solve the problems that ordinary metformin hydrochloride tablets cannot exert the hypoglycemic effect well and are inconvenient to take, and achieve the effect of inhibiting liver and kidney sugars. The effect of xenobiotic effect, reducing the frequency of taking, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
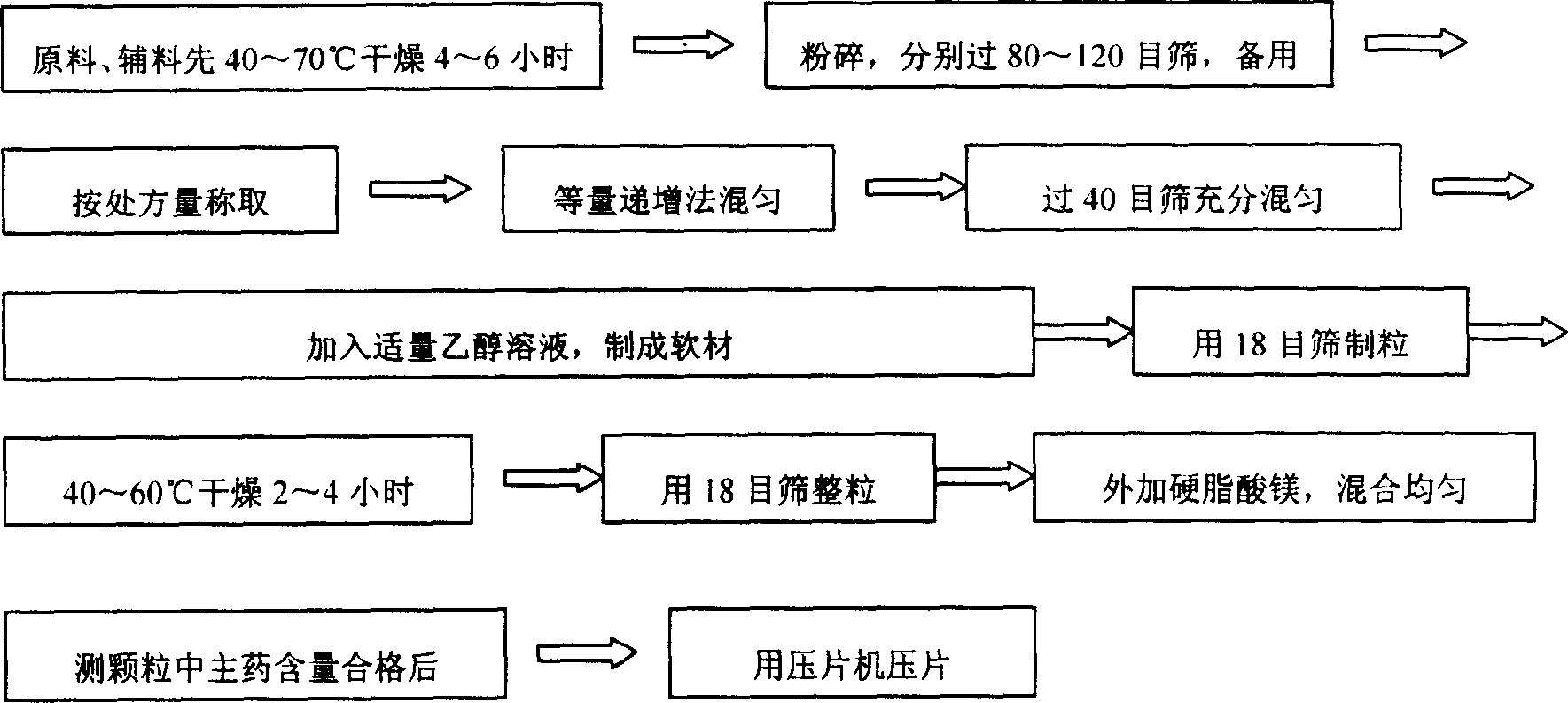
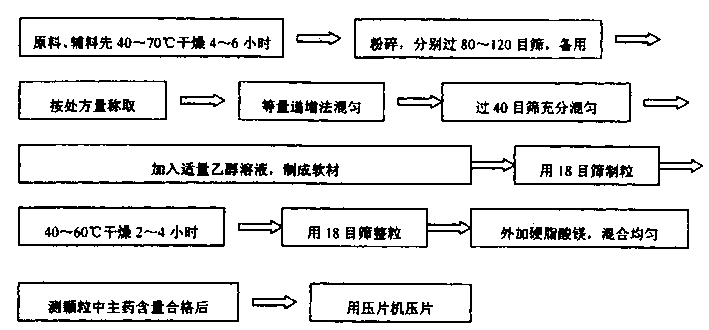
[0021] First, the raw materials and auxiliary materials were first dried at 50°C for 6 hours, all pulverized and passed through a 100-mesh sieve for later use; then, 500 g of metformin hydrochloride, hydroxypropyl methylcellulose HPMC (K 15M ) 450 grams, 20 grams of polyvinylpyrrolidone (PVP) are fully mixed by the method of increasing in equal amounts, then add an appropriate amount of 1% HPMC (K 15M ) 90% ethanol solution, made into soft material, granulated with 18 mesh sieve, dried at 50°C for 2 hours, taken out, granulated with 18 mesh sieve, added 30 grams of magnesium stearate and mixed evenly, and the main drug content in the measured granule was qualified Afterwards, it is pressed into tablets with a tablet machine, and finally 1,000 tablets of finished medicine can be made.
specific Embodiment approach 2
[0022] First, dry the raw materials and auxiliary materials at 60°C for 4 hours, crush them and pass through an 80-mesh sieve for later use; then, take 500 g of metformin hydrochloride, 440 g of sodium carboxymethyl cellulose, and 30 g of ethyl cellulose to fully After mixing, add an appropriate amount of 2% HPMC (K 100M ) 90% ethanol solution to make soft material, granulate with 18 mesh sieve, dry at 50°C for 2 hours, take out, granulate with 18 mesh sieve, add 30 grams of talcum powder and mix evenly, measure the main drug content in the granules after passing the test The tablet press is used to compress the tablets, and finally it can be made into 1000 tablets of finished medicine.
[0023] According to the relevant format and requirements of the 2000 edition of the Pharmacopoeia of the People's Republic of China, use 10% sodium nitroferricyanide solution-sodium ferricyanide test solution-10% sodium hydroxide solution, identification reaction of cyanide and ultraviolet sp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com