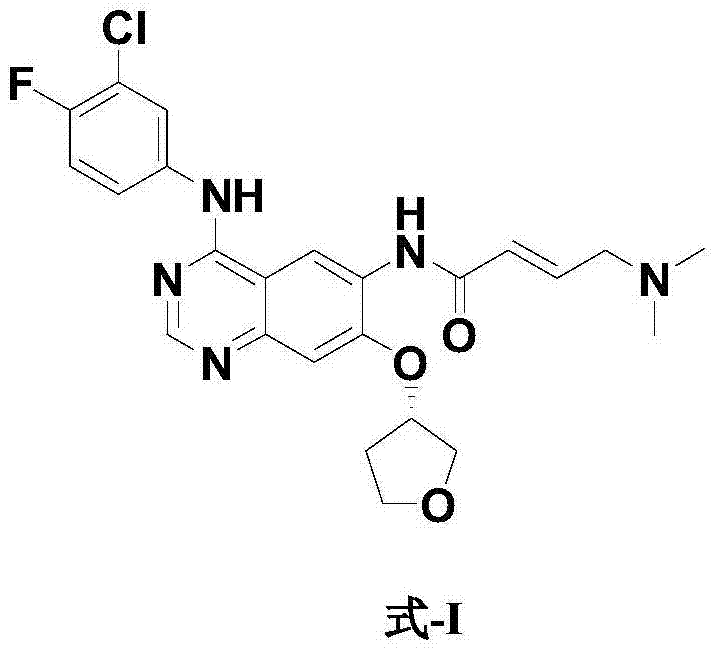
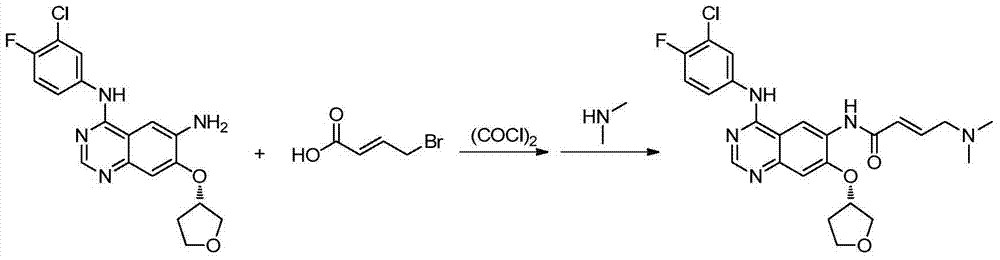
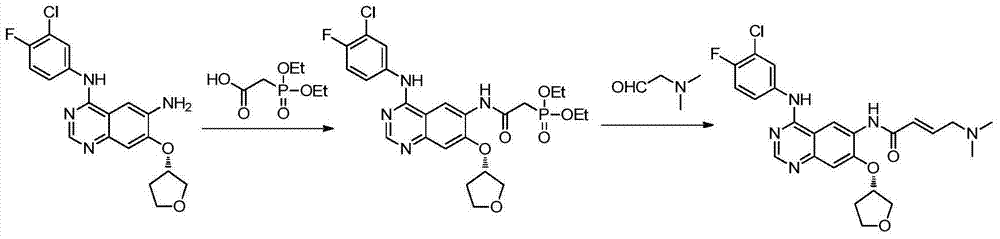
Preparation method for afatinib compound
A technology for afatinib and compounds, applied in the field of chemical synthesis, can solve the problems of unsuitability for large-scale industrial production, high production cost, low total yield, etc., and achieves suitable industrial production, low production cost, and low environmental pollution. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Heat 76mL of diethyl malonate and 150mL of dimethylaminoacetaldehyde diethyl acetal at 105±5°C for 4 hours, TLC detects that the reaction is complete, cool to room temperature, add 150mL of n-heptane and stir evenly, at 0±5°C Cool and crystallize for 12 hours, filter and drain to obtain 99.6 g of condensate with a yield of 86.9%.
Embodiment 2
[0042] Embodiment 2 Preparation of dimethylaminocroton hydrochloride
[0043] Add 95g of the condensate obtained in Example 1 into 800mL of 6N hydrochloric acid aqueous solution, heat to reflux for 10 hours, TLC detects that the reaction is complete, cool and crystallize at 5±5°C for 6 hours, filter and drain, and the obtained solid is at 45±5°C After vacuum drying for 12 hours, 57.7 g of dimethylaminocroton hydrochloride was obtained, with a yield of 84.1%.
Embodiment 3
[0044] Example 3 Synthesis of Afatinib
[0045] Add 53.8g of 1,1-carbonyldiimidazole into 500mL of anhydrous tetrahydrofuran, heat to 40°C to dissolve, add 55g of dimethylaminocroton hydrochloride, react at 40°C for 30 minutes, and set aside.
[0046] 103.7g N 4-(3-Chloro-4-fluoro-phenyl)-7-((S)-tetrahydrofuran-3-yloxy)quinazoline-4,6-diamine was added to 500 mL of anhydrous tetrahydrofuran, and the above solution was added, React at 30°C for 2 hours, TLC detects that the reaction is complete, cool to 10±5°C, and add 1% aqueous sodium hydroxide solution dropwise to the reaction solution at this temperature until the pH is 8-9, add 8L of purified water, and precipitate a large amount of solid , filtered, washed the solid with water until the filtered water was neutral, and dried to obtain 117.2 g of crude product, with a yield of 87.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com