Preparation method of afatinib compound
An afatinib and compound technology, which can be applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., and can solve the problems of high cost oxidative degradation, unfavorable industrial production, difficult long-term storage, etc. problem, to achieve the effect of convenient long-term storage, low cost and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
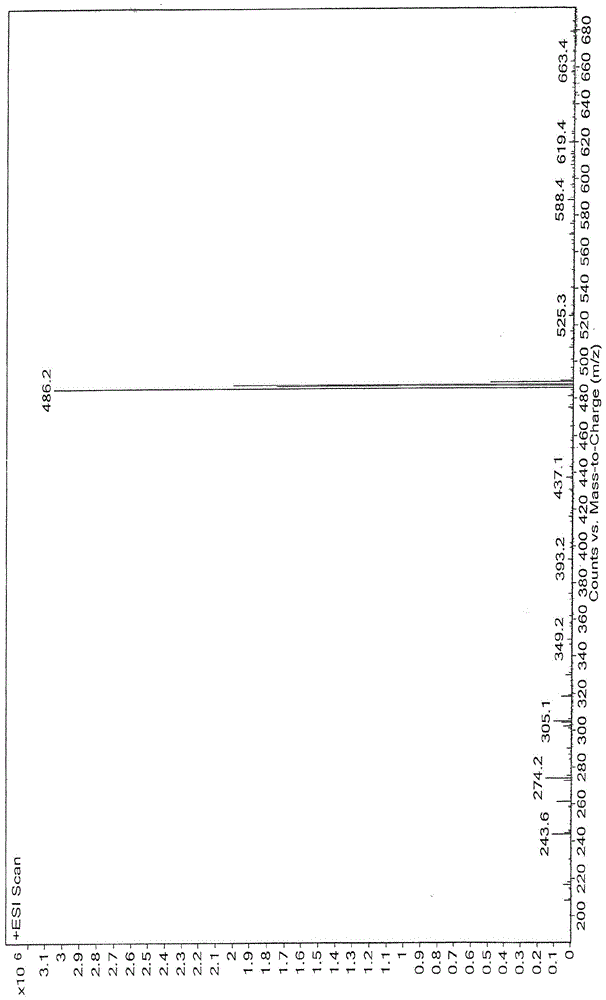
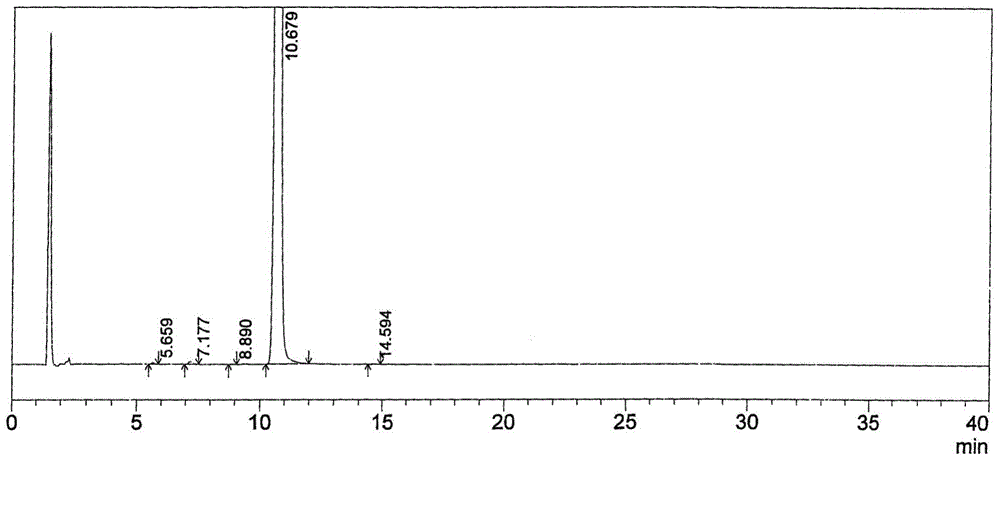
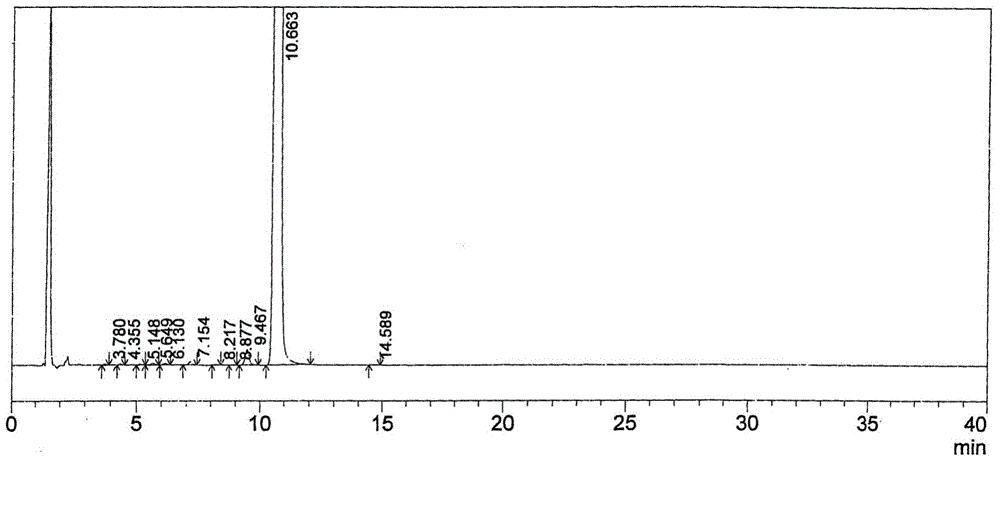
Image
Examples
Embodiment 1
[0037] Embodiment 1 Formula V compound preparation
[0038]
[0039] Phosphorus trichloride (0.5g) was slowly dropped into diphenylphosphineacetic acid (2.37g) dissolved in 25mL of dichloromethane solution, and the reaction temperature was controlled at 0°C. After the dropwise reaction was stirred at 15°C for 2 hours, it was cooled to 0°C. Triethylamine (1.02g) and 4-[(3-chloro-4-fluorophenyl)-amino]-6-amino-7-((S)-tetrahydrofuran-3-yloxy)-quinazoline (3.41g) was dissolved in 25mL of 1,2-dichloroethane solution and slowly added dropwise to the above solution, after the drop was completed, the reaction was carried out at 25°C for 2 hours. After the reaction was monitored by TLC, 20 mL of purified water was added to the extraction layer, and the organic phase was washed with 20 mL of purified water, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to an oil, and tetrahydrofuran / isopropyl ether (1:1) was added. 40 mL was...
Embodiment 2
[0042]Example 2 Preparation of Formula III Compound
[0043]
[0044] n-Butyllithium (1.5M, 6ml) was slowly dropped into the compound of formula V (5g) in 28mL of dry tetrahydrofuran solution at a controlled internal temperature of 0°C. After dropping, cool to -10°C; drop ethyl dimethylaminoacetate (1.05g) into the above solution, after dropping, rise to room temperature and stir for 0.5 hours. After the reaction is monitored by TLC, add saturated chloride Ammonium solution 12mL, saturated sodium chloride solution 8mL, dichloromethane 12mL for extraction and layering, the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure to obtain an oil, which was recrystallized by adding tetrahydrofuran / isopropyl ether (1:2) 40mL An off-white solid (compound of formula III) was obtained.
[0045] n-Butyllithium (1.5M, 6ml) was slowly dropped into the compound of formula V (5g) dissolved in 28mL of dry 2-methyltetrahydrofuran solut...
Embodiment 3
[0047] Embodiment 3 Formula II compound preparation
[0048]
[0049] Add sodium borohydride (0.15g) into a solution of compound of formula III (5g) dissolved in 50mL of methanol, heat at 75°C for 0.5 hours and cool to room temperature, add 50mL of saturated ammonium chloride solution, distill off part of the solvent under reduced pressure, add 50mL Saturated sodium chloride solution, 80mL of dichloromethane for extraction and layering, the organic phase was dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure, the residue was added with 50mL of dichloromethane / n-heptane (1:3) and stirred for crystallization to obtain light yellow Solid (compound of formula II).
[0050] Add sodium borohydride (0.27g) into a solution of compound of formula III (5g) dissolved in 50mL of isopropanol, heat and reflux at 65°C for 4 hours, then cool to room temperature, add 50mL of saturated ammonium chloride solution, and distill off part of the solvent unde...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com