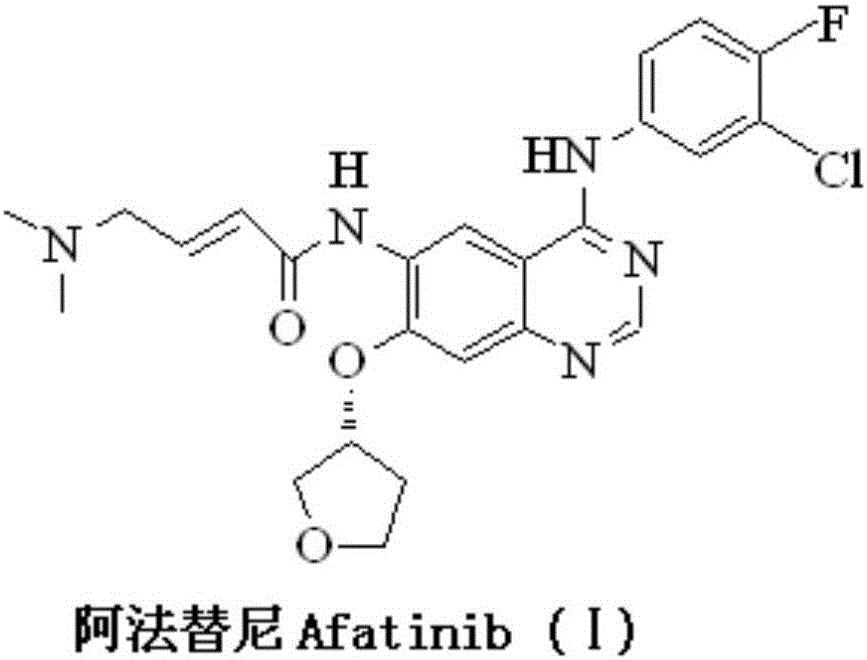
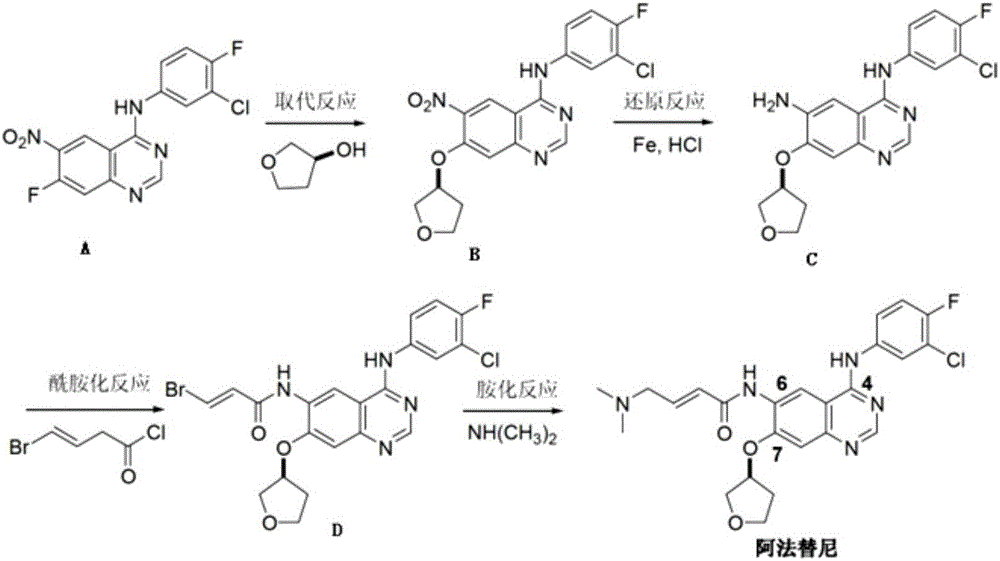
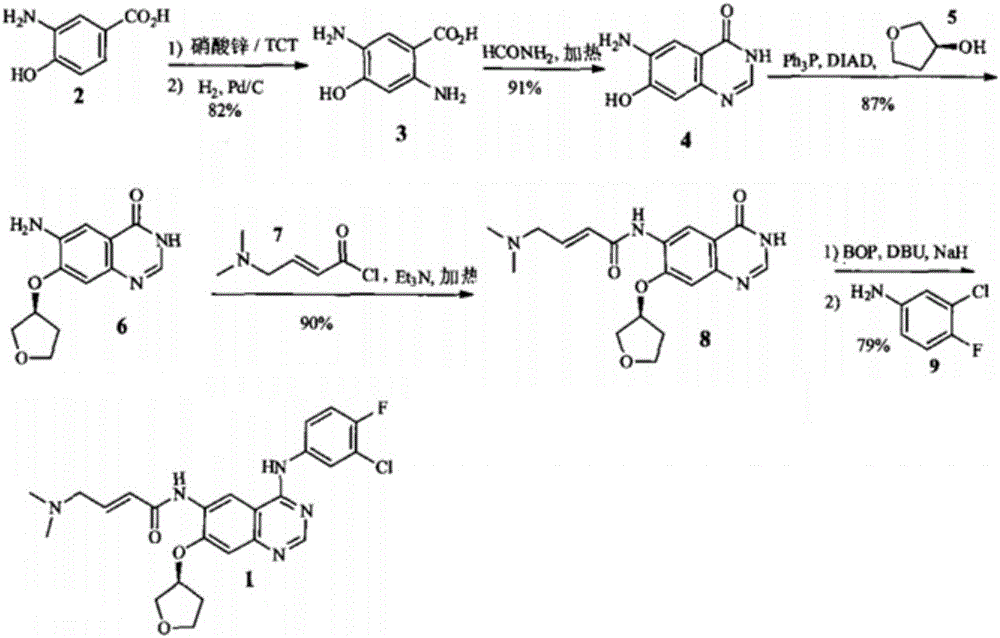
Synthesis method of anti-tumor medicine afatinib
An anti-tumor drug, afatinib technology, applied in the direction of anti-tumor drugs, drug combinations, organic chemistry, etc., can solve the problem of low total yield and achieve the effect of simplified process route, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of compound Ⅲ
[0034] Add 21.8g of compound II, 10.4g of formamidine acetate, 91.6g of tris(dibenzylideneacetone)dipalladium, 2-di-tert-butylphosphine-2',4',6'-triisopropyl Dimethoxy-3,6-dimethoxy-1,1′-biphenyl 96.94g, cesium carbonate 32.6g, n-butanol 2L, react at 85°C for 2h, TLC monitors that the reaction of the raw materials is complete, and naturally cools down to room temperature , filtered, and separated by column chromatography to obtain 16.4 g of compound III with a molar yield of 90% and an HPLC purity of 99.2%.
Embodiment 2
[0036] Preparation of compound Ⅲ
[0037] Add 21.8g of compound II, 11.5g of formamidine acetate, 374.7g of tris(dibenzylideneacetone)dipalladium, 2-di-tert-butylphosphine-2',4',6'-triisopropyl Base-3,6-dimethoxy-1,1′-biphenyl 290.8g, cesium carbonate 71.7g, methanol 2L, reacted at a temperature of 85°C for 2h, TLC monitored the reaction of the raw materials, naturally cooled to room temperature, filtered 17.1 g of compound III was obtained by column chromatography with a molar yield of 94% and a HPLC purity of 99.5%.
Embodiment 3
[0039] Preparation of compound Ⅲ
[0040] Add 21.8g of compound II, 11.5g of formamidine acetate, 374.7g of tris(dibenzylideneacetone)dipalladium, 2-(dicyclohexylphosphine)-3,6-dimethoxy-2' to the reaction flask , 322.1g of 4′,6′-triisopropyl-1,1′-biphenyl, 71.7g of cesium carbonate, 2L of n-butanol, and reacted for 2h at a temperature of 85°C. The raw materials were completely reacted by TLC, and naturally dropped to After filtration at room temperature, 16.2 g of compound III was obtained by column chromatography, with a molar yield of 88% and an HPLC purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com